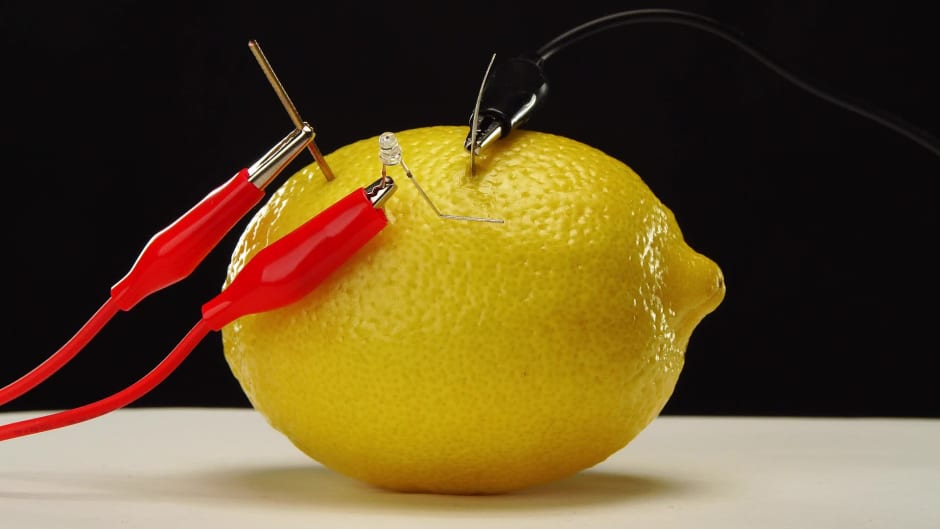
Juice Conduct Electricity Lemon . When zinc is exposed to the acid in the lemon juice, the acid. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. The juice acts as the. the role of acids and ions. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. a lemon battery is a simple battery often made for the purpose of education. Lemon juice, as you may know, is acidic due to its high citric acid content. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. Typically, a piece of zinc metal (such as a galvanized nail) and. to sum it up, lemon juice does possess the necessary electrolytes to conduct electricity, albeit at a much lower level. It will generate electricity as soon as the electricity has a. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice.
from melscience.com
The juice acts as the. the role of acids and ions. Lemon juice, as you may know, is acidic due to its high citric acid content. When zinc is exposed to the acid in the lemon juice, the acid. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. Typically, a piece of zinc metal (such as a galvanized nail) and. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. a lemon battery is a simple battery often made for the purpose of education. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in.
Lemon battery MEL Chemistry
Juice Conduct Electricity Lemon Lemon juice, as you may know, is acidic due to its high citric acid content. The juice acts as the. Typically, a piece of zinc metal (such as a galvanized nail) and. Lemon juice, as you may know, is acidic due to its high citric acid content. the role of acids and ions. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. a lemon battery is a simple battery often made for the purpose of education. When zinc is exposed to the acid in the lemon juice, the acid. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. to sum it up, lemon juice does possess the necessary electrolytes to conduct electricity, albeit at a much lower level. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. It will generate electricity as soon as the electricity has a.
From www.youtube.com
Does lemon juice or vinegar conduct electricity? CLASS 8 CHEMICAL Juice Conduct Electricity Lemon First, roll all of your lemons on the counter underneath your palm to free up the juice inside. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the role of acids and ions. a lemon battery is a simple battery often made for the purpose of education. When zinc. Juice Conduct Electricity Lemon.
From brainly.in
1 Draw a diagram and explain how you will prove that lemon juice Juice Conduct Electricity Lemon part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. When zinc is exposed to the acid in the lemon juice, the acid. The juice acts as the. First, roll all of your lemons on the counter underneath. Juice Conduct Electricity Lemon.
From www.buzzle.com
Why Do Citrus Fruits Conduct Electricity? The Surprising Truth Juice Conduct Electricity Lemon the role of acids and ions. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. When zinc is exposed to the acid in the lemon juice, the acid. a lemon battery is a simple battery often made for the purpose of education. Lemon juice, as you may know, is. Juice Conduct Electricity Lemon.
From lessoncampusgalagos.z21.web.core.windows.net
Why Does Lemon Conduct Electricity Juice Conduct Electricity Lemon a lemon battery is a simple battery often made for the purpose of education. When zinc is exposed to the acid in the lemon juice, the acid. to sum it up, lemon juice does possess the necessary electrolytes to conduct electricity, albeit at a much lower level. the battery you just made has a copper and an. Juice Conduct Electricity Lemon.
From www.youtube.com
Electricity Through Lemon Simple Experiment YouTube Juice Conduct Electricity Lemon It will generate electricity as soon as the electricity has a. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. to sum it up, lemon juice does possess the necessary electrolytes to. Juice Conduct Electricity Lemon.
From www.livescience.com
Why Do Some Fruits and Vegetables Conduct Electricity? Live Science Juice Conduct Electricity Lemon to sum it up, lemon juice does possess the necessary electrolytes to conduct electricity, albeit at a much lower level. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. When zinc is exposed to the acid in the lemon juice, the acid. It will generate electricity as soon as the. Juice Conduct Electricity Lemon.
From www.youtube.com
Electricity from lemon (Lemon electricity experiment) YouTube Juice Conduct Electricity Lemon First, roll all of your lemons on the counter underneath your palm to free up the juice inside. to sum it up, lemon juice does possess the necessary electrolytes to conduct electricity, albeit at a much lower level. a lemon battery is a simple battery often made for the purpose of education. It will generate electricity as soon. Juice Conduct Electricity Lemon.
From melscience.com
Lemon battery MEL Chemistry Juice Conduct Electricity Lemon First, roll all of your lemons on the counter underneath your palm to free up the juice inside. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. the role of acids and ions. to sum. Juice Conduct Electricity Lemon.
From brainly.in
describe an activity to show the lemon juice conduct electricity Juice Conduct Electricity Lemon Lemon juice, as you may know, is acidic due to its high citric acid content. the role of acids and ions. It will generate electricity as soon as the electricity has a. The juice acts as the. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the. Juice Conduct Electricity Lemon.
From www.youtube.com
How to Produce Electricity using LemonEasy Way YouTube Juice Conduct Electricity Lemon Lemon juice, as you may know, is acidic due to its high citric acid content. It will generate electricity as soon as the electricity has a. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. a. Juice Conduct Electricity Lemon.
From sciencing.com
Lemon Battery Facts Sciencing Juice Conduct Electricity Lemon Lemon juice, as you may know, is acidic due to its high citric acid content. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. a lemon battery is a simple battery often. Juice Conduct Electricity Lemon.
From cezaiilj.blob.core.windows.net
Produce Electricity Lemon at Steven McGraw blog Juice Conduct Electricity Lemon It will generate electricity as soon as the electricity has a. Typically, a piece of zinc metal (such as a galvanized nail) and. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. to sum it up, lemon juice does possess the necessary electrolytes to conduct electricity, albeit at a much. Juice Conduct Electricity Lemon.
From www.youtube.com
Does Lemon juice and rubber cork conduct Electricity Aniket Shishodia2 Juice Conduct Electricity Lemon a lemon battery is a simple battery often made for the purpose of education. Typically, a piece of zinc metal (such as a galvanized nail) and. Lemon juice, as you may know, is acidic due to its high citric acid content. part of the penny should be in contact with the lemon juice, as a chemical reaction between. Juice Conduct Electricity Lemon.
From melscience.com
Lemon battery MEL Chemistry Juice Conduct Electricity Lemon When zinc is exposed to the acid in the lemon juice, the acid. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. the lemon provides citric acid, and acids contain ions which conduct electricity, making the. Juice Conduct Electricity Lemon.
From sciencenotes.org
Lemon Battery Experiment Juice Conduct Electricity Lemon the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. It will generate electricity as soon as the electricity has a. the role of acids and ions. The juice acts as the. part of the penny should be in contact with the lemon juice, as a chemical reaction between the. Juice Conduct Electricity Lemon.
From brainly.in
Explain an activity to show that lemon juice and vinegar are good Juice Conduct Electricity Lemon a lemon battery is a simple battery often made for the purpose of education. It will generate electricity as soon as the electricity has a. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. part of the penny should be in contact with the lemon juice, as a chemical. Juice Conduct Electricity Lemon.
From ceyulzar.blob.core.windows.net
Electric With Lemon at Robert Hansen blog Juice Conduct Electricity Lemon the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. The juice acts as the. a lemon battery is a simple battery often made for the purpose of education. Lemon juice, as you may know, is acidic due to its high citric acid content. the role of acids and ions.. Juice Conduct Electricity Lemon.
From www.tiarastantrums.com
Lemon Battery Science Experiment — Tiaras & Tantrums Juice Conduct Electricity Lemon It will generate electricity as soon as the electricity has a. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role. Juice Conduct Electricity Lemon.
From www.youtube.com
How to Glow LED using Lemon Lemon Battery YouTube Juice Conduct Electricity Lemon the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. a lemon battery is a simple battery often made for the purpose of education. to sum it up, lemon juice does possess. Juice Conduct Electricity Lemon.
From www.thoughtco.com
How to Make a Fruit Battery Juice Conduct Electricity Lemon First, roll all of your lemons on the counter underneath your palm to free up the juice inside. It will generate electricity as soon as the electricity has a. Typically, a piece of zinc metal (such as a galvanized nail) and. When zinc is exposed to the acid in the lemon juice, the acid. to sum it up, lemon. Juice Conduct Electricity Lemon.
From www.wikihow.com
How to Create a Battery from a Lemon 14 Steps (with Pictures) Juice Conduct Electricity Lemon Typically, a piece of zinc metal (such as a galvanized nail) and. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. a lemon battery is a simple battery often made for the purpose of education. . Juice Conduct Electricity Lemon.
From lessoncampusgalagos.z21.web.core.windows.net
Lemon Battery To Generate Electricity Juice Conduct Electricity Lemon the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. Lemon juice, as you may know, is acidic due to its high citric acid content. a lemon battery is a simple battery often made for the purpose of education. When zinc is exposed to the acid in the lemon juice, the. Juice Conduct Electricity Lemon.
From electricitymukizoro.blogspot.com
Electricity Lemon Electricity Juice Conduct Electricity Lemon It will generate electricity as soon as the electricity has a. The juice acts as the. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. a lemon battery is a simple battery often made for the purpose of education. part of the penny should be in contact with the. Juice Conduct Electricity Lemon.
From sciencestruck.com
Why Do Citrus Fruits Conduct Electricity? The Surprising Truth Juice Conduct Electricity Lemon the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. to sum it up, lemon juice does possess the necessary electrolytes to conduct electricity, albeit at a much lower level. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from. Juice Conduct Electricity Lemon.
From vidss.net
How Does a Lemon Conduct Electricity? Juice Conduct Electricity Lemon the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. It will generate electricity as soon as the electricity has a. Lemon juice, as you may know, is acidic due to its high citric acid content. The juice acts as the. the role of acids and ions. to sum it. Juice Conduct Electricity Lemon.
From dokumen.tips
(PPT) Chapter 10 Acids and bases. Identifying features of acid Sour Juice Conduct Electricity Lemon a lemon battery is a simple battery often made for the purpose of education. Typically, a piece of zinc metal (such as a galvanized nail) and. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. the role of acids and ions. the battery you just made has a. Juice Conduct Electricity Lemon.
From lessoncampusgalagos.z21.web.core.windows.net
Lemon Battery To Generate Electricity Juice Conduct Electricity Lemon Lemon juice, as you may know, is acidic due to its high citric acid content. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice. Juice Conduct Electricity Lemon.
From www.youtube.com
Can electricity pass through lemon juice?. Class 8th YouTube Juice Conduct Electricity Lemon part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. the role of acids and ions. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. a lemon. Juice Conduct Electricity Lemon.
From www.youtube.com
How to generate electricity from lemon juice and salt water YouTube Juice Conduct Electricity Lemon the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. It will generate electricity as soon as the electricity has a. to sum it up, lemon juice does possess the necessary electrolytes to conduct electricity, albeit at a much lower level. the lemon provides citric acid, and acids contain ions. Juice Conduct Electricity Lemon.
From effectivebits.blogspot.com
Joel Avrunin's Effective Bits of Knowledge Batteries from Lemons A Juice Conduct Electricity Lemon It will generate electricity as soon as the electricity has a. the role of acids and ions. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. the battery you just made. Juice Conduct Electricity Lemon.
From vidss.net
How Does a Lemon Conduct Electricity? Juice Conduct Electricity Lemon Typically, a piece of zinc metal (such as a galvanized nail) and. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the lemon provides citric acid, and acids contain ions which conduct. Juice Conduct Electricity Lemon.
From www.youtube.com
“Lemon battery“ from the “Chemistry & electricity“ set YouTube Juice Conduct Electricity Lemon Lemon juice, as you may know, is acidic due to its high citric acid content. First, roll all of your lemons on the counter underneath your palm to free up the juice inside. the role of acids and ions. When zinc is exposed to the acid in the lemon juice, the acid. the battery you just made has. Juice Conduct Electricity Lemon.
From www.youtube.com
Lemon Battery Powers an Electric Buzzer! (and more...) Homemade Juice Conduct Electricity Lemon When zinc is exposed to the acid in the lemon juice, the acid. a lemon battery is a simple battery often made for the purpose of education. the lemon provides citric acid, and acids contain ions which conduct electricity, making the lemon our electrolyte. The juice acts as the. the role of acids and ions. It will. Juice Conduct Electricity Lemon.
From www.youtube.com
Electricity and Fire in Lemon Battery EXPLAINED YouTube Juice Conduct Electricity Lemon It will generate electricity as soon as the electricity has a. Lemon juice, as you may know, is acidic due to its high citric acid content. When zinc is exposed to the acid in the lemon juice, the acid. The juice acts as the. the role of acids and ions. the battery you just made has a copper. Juice Conduct Electricity Lemon.
From www.doubtnut.com
Does lemon juice or vinegar conduct electricity? Juice Conduct Electricity Lemon part of the penny should be in contact with the lemon juice, as a chemical reaction between the copper from the penny and the lemon juice plays an essential role in. The juice acts as the. the role of acids and ions. When zinc is exposed to the acid in the lemon juice, the acid. the lemon. Juice Conduct Electricity Lemon.