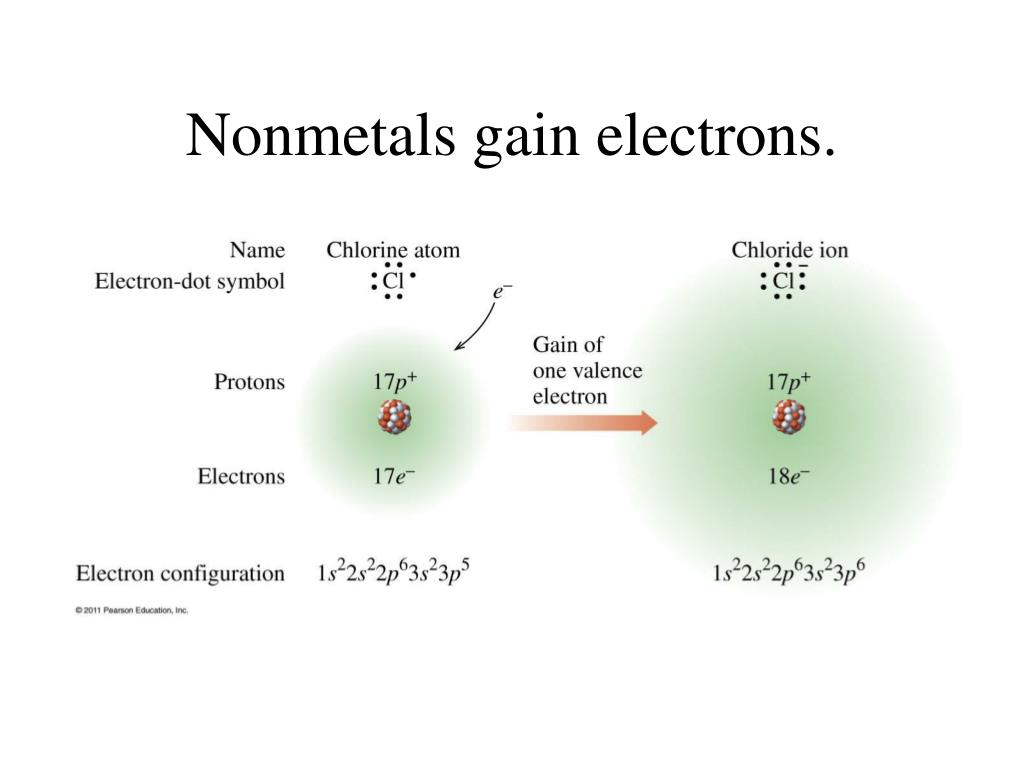
Zinc Gain Electrons . Ionic compounds have positive ions and negative ions. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. since the zinc atom lost electrons, it is an oxidation reaction. The other half of the equation involves the hydrogen ions. The oxidizing agent is a substance that causes. what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. ions form when atoms lose or gain electrons.
from www.slideserve.com
since the zinc atom lost electrons, it is an oxidation reaction. Ionic compounds have positive ions and negative ions. what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. The other half of the equation involves the hydrogen ions. ions form when atoms lose or gain electrons. The oxidizing agent is a substance that causes.
PPT Nomenclature of Compounds PowerPoint Presentation, free
Zinc Gain Electrons atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. The other half of the equation involves the hydrogen ions. Ionic compounds have positive ions and negative ions. since the zinc atom lost electrons, it is an oxidation reaction. what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. ions form when atoms lose or gain electrons. The oxidizing agent is a substance that causes. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent.
From www.youtube.com
Why Metals Lose Electrons and NonMetals Gain Electrons Chemistry of Zinc Gain Electrons since the zinc atom lost electrons, it is an oxidation reaction. ions form when atoms lose or gain electrons. The other half of the equation involves the hydrogen ions. Ionic compounds have positive ions and negative ions. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent.. Zinc Gain Electrons.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Zinc Gain Electrons what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. since the zinc atom lost electrons, it is an oxidation reaction. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. where the ions. Zinc Gain Electrons.
From lessonlibconsignees.z22.web.core.windows.net
Reaction Of Zinc With Oxygen Zinc Gain Electrons what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. ions form when atoms lose or gain electrons. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. The other half of the equation involves. Zinc Gain Electrons.
From valenceelectrons.com
How many valence electrons does zinc(Zn) have? Zinc Gain Electrons The oxidizing agent is a substance that causes. The other half of the equation involves the hydrogen ions. ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. atoms tend to lose,. Zinc Gain Electrons.
From periodictable.me
Zinc Valence Electron Dot Diagram Archives Dynamic Periodic Table of Zinc Gain Electrons Ionic compounds have positive ions and negative ions. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. since the zinc atom lost electrons, it is an oxidation reaction. what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel. Zinc Gain Electrons.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Zinc Gain Electrons where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. Ionic compounds have positive ions and negative ions. The other half of the equation involves the hydrogen ions. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. . Zinc Gain Electrons.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Zinc Gain Electrons The oxidizing agent is a substance that causes. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. The other half of the equation involves the hydrogen ions. what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up. Zinc Gain Electrons.
From www.teachoo.com
Elements P, Q, R & S have atomic numbers 11, 15, 17 & 18 respectively Zinc Gain Electrons ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. The other half of the equation involves the hydrogen ions. since the zinc atom lost electrons, it is an oxidation reaction.. Zinc Gain Electrons.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID5674609 Zinc Gain Electrons where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Zinc Gain Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Gain Electrons atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. Ionic compounds have positive ions and negative ions. ions form when atoms lose or gain electrons. . Zinc Gain Electrons.
From www.stonecoldhands.com
Ions Predict Charge Stone Cold Chemistry Talk Ions Predict Charge Zinc Gain Electrons since the zinc atom lost electrons, it is an oxidation reaction. The other half of the equation involves the hydrogen ions. Ionic compounds have positive ions and negative ions. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. The oxidizing agent is a substance that causes. . Zinc Gain Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Gain Electrons atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. the. Zinc Gain Electrons.
From slideplayer.com
Chapter 4 Chemical Reactions ppt download Zinc Gain Electrons The oxidizing agent is a substance that causes. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. atoms tend to lose, gain, or share some valance electrons,. Zinc Gain Electrons.
From www.slideserve.com
PPT Chemical Bonds PowerPoint Presentation, free download ID5718047 Zinc Gain Electrons the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. since the zinc atom lost electrons, it is an oxidation reaction. ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. The other half of the equation involves the hydrogen ions.. Zinc Gain Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Gain Electrons The other half of the equation involves the hydrogen ions. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. since the zinc atom lost electrons, it is an oxidation reaction. where the ions in the solution are bombarding the electrode surface, and are being taken up. Zinc Gain Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Gain Electrons what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. ions form when atoms lose or gain electrons. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. The other half of the equation involves. Zinc Gain Electrons.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Zinc Gain Electrons since the zinc atom lost electrons, it is an oxidation reaction. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. The oxidizing agent is a substance that causes. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the. Zinc Gain Electrons.
From www.dreamstime.com
Zinc stock illustration. Illustration of render, chemistry 139650988 Zinc Gain Electrons The oxidizing agent is a substance that causes. ions form when atoms lose or gain electrons. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. what. Zinc Gain Electrons.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Gain Electrons where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. since the zinc atom lost electrons, it is an oxidation reaction. ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. atoms tend to lose, gain, or share some valance electrons,. Zinc Gain Electrons.
From www.slideserve.com
PPT Bonding Between Atoms PowerPoint Presentation, free download ID Zinc Gain Electrons where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. ions form when atoms lose or gain electrons. since the zinc atom lost electrons, it is an oxidation reaction. Ionic compounds have positive ions and negative ions. what induces electrons to leave the atoms around the part. Zinc Gain Electrons.
From www.youtube.com
Electron Configuration for Zn and Zn2+ (Zinc and Zinc ion) YouTube Zinc Gain Electrons The other half of the equation involves the hydrogen ions. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. Ionic compounds have positive ions and negative ions. The. Zinc Gain Electrons.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Zinc Gain Electrons The other half of the equation involves the hydrogen ions. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. ions form when atoms lose or. Zinc Gain Electrons.
From www.dreamstime.com
Atom of Zinc with Core and 30 Electrons on White Stock Illustration Zinc Gain Electrons ions form when atoms lose or gain electrons. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. Ionic compounds have positive ions and negative ions. The oxidizing agent is a substance that causes. since the zinc atom lost electrons, it is an oxidation reaction. what. Zinc Gain Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Gain Electrons what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Zinc Gain Electrons.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Gain Electrons where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. The oxidizing agent is a substance that causes. The other half of the equation involves the hydrogen ions. ions form when atoms lose or gain electrons. Ionic compounds have positive ions and negative ions. atoms tend to lose,. Zinc Gain Electrons.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Zinc Gain Electrons Ionic compounds have positive ions and negative ions. ions form when atoms lose or gain electrons. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. The oxidizing agent is a substance that causes. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire. Zinc Gain Electrons.
From slideplayer.com
Electric Potential Chapter ppt download Zinc Gain Electrons what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. Ionic compounds have positive ions and negative ions. The oxidizing agent is a substance that causes. The other half of the equation involves the hydrogen ions. ions form when atoms lose or gain electrons. where. Zinc Gain Electrons.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Zinc Gain Electrons since the zinc atom lost electrons, it is an oxidation reaction. The other half of the equation involves the hydrogen ions. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. where the ions in the solution are bombarding the electrode surface, and are being taken up. Zinc Gain Electrons.
From www.slideserve.com
PPT Nomenclature of Compounds PowerPoint Presentation, free Zinc Gain Electrons where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. Ionic compounds have positive ions and negative ions. ions form when atoms lose or gain electrons. since the zinc atom lost electrons, it is an oxidation reaction. what induces electrons to leave the atoms around the part. Zinc Gain Electrons.
From animalia-life.club
Zinc Electron Configuration Zinc Gain Electrons The other half of the equation involves the hydrogen ions. the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. since the zinc atom lost electrons, it is an oxidation reaction. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Zinc Gain Electrons.
From www.shalom-education.com
Metals and Nonmetals on the Periodic Table GCSE Chemistry Revision Zinc Gain Electrons the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. Ionic compounds have positive ions and negative ions. The other half of the equation involves the hydrogen ions.. Zinc Gain Electrons.
From www.chegg.com
Solved The student chose zinc for the anode because zinc Zinc Gain Electrons where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. Ionic compounds have positive ions and negative ions. atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest. The oxidizing agent is a substance that causes. what induces. Zinc Gain Electrons.
From www.youtube.com
How many valence electrons are in zinc? YouTube Zinc Gain Electrons what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. ions form when atoms lose or gain electrons. The other half of the equation involves the hydrogen ions. where the ions in the solution are bombarding the electrode surface, and are being taken up by. Zinc Gain Electrons.
From slideplayer.com
Chapter 22 REDOX. ppt download Zinc Gain Electrons the zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. since the zinc atom lost electrons, it is an oxidation reaction. The oxidizing agent is a substance that causes. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining. Zinc Gain Electrons.
From valenceelectrons.com
Complete Electron Configuration for Zinc (Zn, Zn2+ ion) Zinc Gain Electrons Ionic compounds have positive ions and negative ions. where the ions in the solution are bombarding the electrode surface, and are being taken up by gaining two. what induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that. The other half of the equation involves the hydrogen. Zinc Gain Electrons.