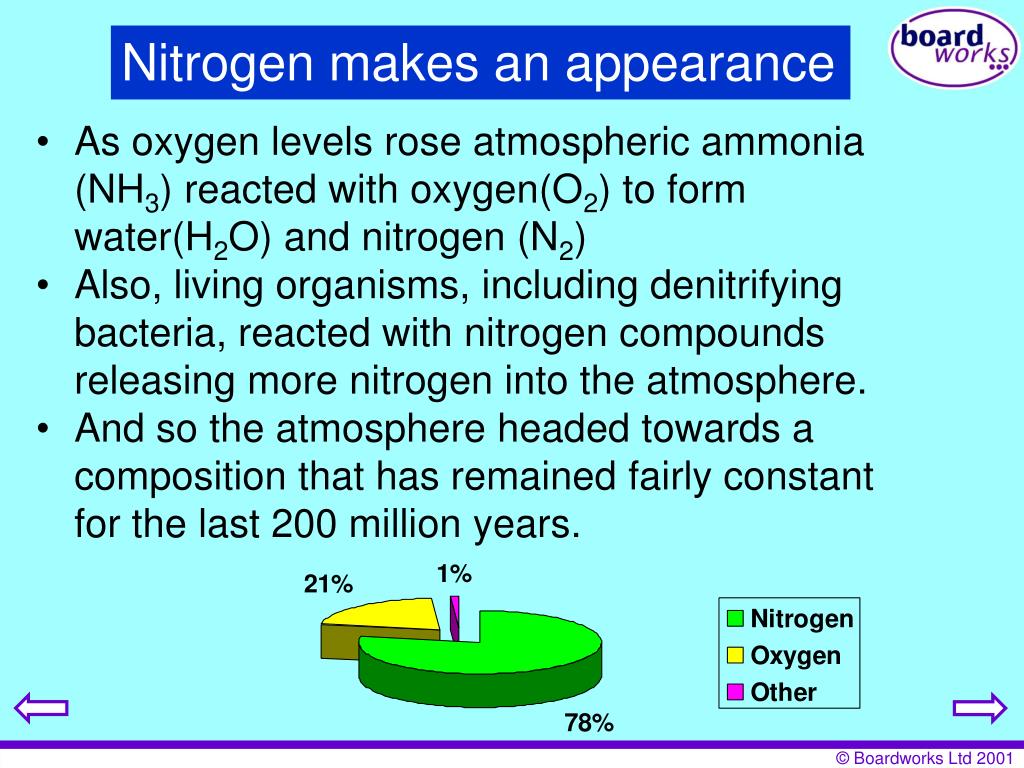
Nitrogen In The Atmosphere Is 99.63 . The mass defect for the nucleus of helium is 0.0303 amu. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). 14n is the more abundant of the two, comprising 99.63%. Given this information, determine the atomic mass of. Atomic mass of nitrogen = 14.0067 amu. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). Given this information, determine the atomic mass of. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). The binding energy per nucleon in m ev is. Isotopes can be defined as two or more forms of a chemical element that. Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively.
from www.slideserve.com
Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. 14n is the more abundant of the two, comprising 99.63%. The mass defect for the nucleus of helium is 0.0303 amu. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). Isotopes can be defined as two or more forms of a chemical element that. The binding energy per nucleon in m ev is. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). Atomic mass of nitrogen = 14.0067 amu. Given this information, determine the atomic mass of.
PPT KS4 Changes to the Earth and atmosphere PowerPoint Presentation
Nitrogen In The Atmosphere Is 99.63 Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Atomic mass of nitrogen = 14.0067 amu. The binding energy per nucleon in m ev is. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). 14n is the more abundant of the two, comprising 99.63%. The mass defect for the nucleus of helium is 0.0303 amu. Given this information, determine the atomic mass of. Given this information, determine the atomic mass of. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). Isotopes can be defined as two or more forms of a chemical element that.
From www.pinterest.com
Composition of atmosphere Atmosphere, Nitrogen, Dust particles Nitrogen In The Atmosphere Is 99.63 The mass defect for the nucleus of helium is 0.0303 amu. If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Nitrogen in the atmosphere is 99.63% 'n (14.00307. Nitrogen In The Atmosphere Is 99.63.
From byjus.com
Nitrogen Cycle Explained Definition, Stages and Importance Nitrogen In The Atmosphere Is 99.63 Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). The mass defect for the nucleus of helium is 0.0303 amu. 14n is the more abundant of the two, comprising 99.63%. Nitrogen in the atmosphere. Nitrogen In The Atmosphere Is 99.63.
From www.youtube.com
Why Nitrogen is the most abundant gas on earth atmosphere YouTube Nitrogen In The Atmosphere Is 99.63 Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). The mass defect for the nucleus of helium is 0.0303 amu. Atomic mass of nitrogen = 14.0067 amu. The binding energy per nucleon in m ev is. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). Nitrogen in the atmosphere. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT Nitrogen gas returns to the atmosphere by the action of Nitrogen In The Atmosphere Is 99.63 14n is the more abundant of the two, comprising 99.63%. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Isotopes can be defined as two or more forms of a chemical element that. Given this information, determine the atomic mass of. The binding energy per nucleon in m ev is. Atomic mass of nitrogen =. Nitrogen In The Atmosphere Is 99.63.
From www.teachoo.com
Nitrogen Cycle Diagram with Steps Explained Teachoo Concepts Nitrogen In The Atmosphere Is 99.63 Isotopes can be defined as two or more forms of a chemical element that. The mass defect for the nucleus of helium is 0.0303 amu. Given this information, determine the atomic mass of. Atomic mass of nitrogen = 14.0067 amu. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). If average mass of nitrogen is. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID Nitrogen In The Atmosphere Is 99.63 Isotopes can be defined as two or more forms of a chemical element that. Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). If average mass of nitrogen is 14.0067, calculate the relative. Nitrogen In The Atmosphere Is 99.63.
From dokumen.tips
(PPT) What is Nitrogen? Nitrogen makes up about 78 of our atmosphere Nitrogen In The Atmosphere Is 99.63 If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). Given this information, determine the atomic mass of. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). The mass defect for. Nitrogen In The Atmosphere Is 99.63.
From www.britannica.com
nitrogen Definition, Symbol, Uses, Properties, Atomic Number, & Facts Nitrogen In The Atmosphere Is 99.63 Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). The mass defect for the nucleus of helium is 0.0303 amu. Isotopes can be defined as two or more forms of a chemical element that. Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively.. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation ID7047601 Nitrogen In The Atmosphere Is 99.63 Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). The binding energy per nucleon in m ev is. 14n is the more abundant of the two, comprising 99.63%. Given this information, determine the atomic. Nitrogen In The Atmosphere Is 99.63.
From www.britannica.com
How the Nitrogen Cycle Works Britannica Nitrogen In The Atmosphere Is 99.63 Given this information, determine the atomic mass of. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). Atomic mass of nitrogen = 14.0067 amu. Isotopes can be defined as two or more forms of a chemical element that. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). The mass. Nitrogen In The Atmosphere Is 99.63.
From cartoondealer.com
The Composition Of The Atmosphere. Nitrogen, Carbon Dioxide, Oxygen Nitrogen In The Atmosphere Is 99.63 Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). 14n is the more abundant of the two, comprising 99.63%. Given this information, determine the atomic mass of. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). If average mass of nitrogen is 14.0067, calculate the relative abundance of both. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID722344 Nitrogen In The Atmosphere Is 99.63 Given this information, determine the atomic mass of. The binding energy per nucleon in m ev is. 14n is the more abundant of the two, comprising 99.63%. The mass defect for the nucleus of helium is 0.0303 amu. Atomic mass of nitrogen = 14.0067 amu. Given this information, determine the atomic mass of. Nitrogen in the atmosphere is 99.63% n. Nitrogen In The Atmosphere Is 99.63.
From www.collegesidekick.com
Atmospheric Gasses Physical Geography Nitrogen In The Atmosphere Is 99.63 Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Atomic mass of nitrogen = 14.0067 amu. Given this information, determine the atomic mass of. 14n is the more abundant of the two, comprising 99.63%.. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT Elements on Earth PowerPoint Presentation, free download Nitrogen In The Atmosphere Is 99.63 Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Given this information, determine the atomic mass of. 14n is the more abundant of the two, comprising 99.63%. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Atomic mass of nitrogen = 14.0067 amu.. Nitrogen In The Atmosphere Is 99.63.
From www.earth.com
Why is the Nitrogen Cycle So Important? Nitrogen In The Atmosphere Is 99.63 Atomic mass of nitrogen = 14.0067 amu. Given this information, determine the atomic mass of. The mass defect for the nucleus of helium is 0.0303 amu. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Given this information, determine the atomic mass of. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID Nitrogen In The Atmosphere Is 99.63 Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT Nitrogen Cycle PowerPoint Presentation, free download ID5497134 Nitrogen In The Atmosphere Is 99.63 Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). Atomic mass of nitrogen = 14.0067 amu. Given this information, determine the atomic mass of. The binding energy per. Nitrogen In The Atmosphere Is 99.63.
From www.haikudeck.com
Nitrogen Cycle by Bob Saggins Nitrogen In The Atmosphere Is 99.63 14n is the more abundant of the two, comprising 99.63%. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). The binding energy per nucleon in m ev is. Given this information, determine the atomic mass of. Isotopes can be defined as two or more forms of a chemical element that. The mass defect for the. Nitrogen In The Atmosphere Is 99.63.
From www.earth.com
Why is the Nitrogen Cycle So Important? Nitrogen In The Atmosphere Is 99.63 The mass defect for the nucleus of helium is 0.0303 amu. If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). Given this information, determine the atomic mass of. 14n is the more abundant of the two, comprising 99.63%. Nitrogen has two stable isotopes, 14 n and 15 n, whose. Nitrogen In The Atmosphere Is 99.63.
From www.vectorstock.com
The composition of the atmosphere Nitrogen carbon Vector Image Nitrogen In The Atmosphere Is 99.63 Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). Given this information, determine the atomic mass of. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Isotopes can be defined as two or more forms of a chemical element that. 14n is the more abundant of the two, comprising 99.63%.. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation ID722344 Nitrogen In The Atmosphere Is 99.63 Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). If average mass of nitrogen is 14.0067, calculate the relative. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID Nitrogen In The Atmosphere Is 99.63 The binding energy per nucleon in m ev is. Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Atomic mass of nitrogen = 14.0067 amu. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). If average mass of nitrogen is 14.0067, calculate the. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT NITROGEN CYCLE PowerPoint Presentation, free download ID1992578 Nitrogen In The Atmosphere Is 99.63 Given this information, determine the atomic mass of. If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). Given this information, determine the atomic mass of. The mass defect for the nucleus of helium is 0.0303 amu. 14n is the more abundant of the two, comprising 99.63%. Nitrogen has two. Nitrogen In The Atmosphere Is 99.63.
From www.alamy.com
Air, composition of Earth's atmosphere by volume, excluding water vapor Nitrogen In The Atmosphere Is 99.63 Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). 14n is the more abundant of the two, comprising 99.63%. The binding energy per nucleon in m ev is. Isotopes can be defined as two or more forms of a chemical element that. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n. Nitrogen In The Atmosphere Is 99.63.
From exyjyvsht.blob.core.windows.net
How Is Nitrogen Gas Released Back Into The Atmosphere at Linda Fort blog Nitrogen In The Atmosphere Is 99.63 Given this information, determine the atomic mass of. Isotopes can be defined as two or more forms of a chemical element that. If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). The binding energy per nucleon in m ev is. Nitrogen has two stable isotopes, 14n and 15n (atomic. Nitrogen In The Atmosphere Is 99.63.
From stock.adobe.com
The nitrogen cycle showing relationship between the three main nitrogen Nitrogen In The Atmosphere Is 99.63 The binding energy per nucleon in m ev is. Isotopes can be defined as two or more forms of a chemical element that. Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. The mass defect for the nucleus of helium is 0.0303 amu. Nitrogen in the atmosphere is. Nitrogen In The Atmosphere Is 99.63.
From www.sciencelearn.org.nz
The terrestrial nitrogen cycle — Science Learning Hub Nitrogen In The Atmosphere Is 99.63 The mass defect for the nucleus of helium is 0.0303 amu. Isotopes can be defined as two or more forms of a chemical element that. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Given this information, determine the atomic mass. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT NITROGEN CYCLE PowerPoint Presentation, free download ID1992578 Nitrogen In The Atmosphere Is 99.63 Given this information, determine the atomic mass of. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). 14n is the more abundant of the two, comprising 99.63%. Given this information, determine the atomic mass of. Atomic mass of nitrogen = 14.0067 amu. The mass defect for the nucleus of helium is 0.0303 amu. Nitrogen has. Nitrogen In The Atmosphere Is 99.63.
From studylib.net
Chapter 17 The Atmosphere • Composition of the • 78 Nitrogen Nitrogen In The Atmosphere Is 99.63 The mass defect for the nucleus of helium is 0.0303 amu. Isotopes can be defined as two or more forms of a chemical element that. Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). 14n is the more abundant of the two, comprising 99.63%. The binding energy per nucleon in m ev is. If average. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT The Nitrogen Cycle PowerPoint Presentation, free download ID Nitrogen In The Atmosphere Is 99.63 Atomic mass of nitrogen = 14.0067 amu. 14n is the more abundant of the two, comprising 99.63%. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). The binding energy per nucleon in m ev is. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). Nitrogen in the atmosphere is. Nitrogen In The Atmosphere Is 99.63.
From present5.com
THE NITROGEN CYCLENitrates are essential for plant growth Nitrogen In The Atmosphere Is 99.63 Atomic mass of nitrogen = 14.0067 amu. Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT Evolution of Oxygen in the Atmosphere PowerPoint Presentation Nitrogen In The Atmosphere Is 99.63 Nitrogen in the atmosphere is 99.63% n (14.00307 amu) and 0.37% n (15.00011 amu). Isotopes can be defined as two or more forms of a chemical element that. The binding energy per nucleon in m ev is. Given this information, determine the atomic mass of. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively).. Nitrogen In The Atmosphere Is 99.63.
From www.youtube.com
Why Nitrogen is most abundant gas in atmosphere?and what happens when Nitrogen In The Atmosphere Is 99.63 Given this information, determine the atomic mass of. The binding energy per nucleon in m ev is. Nitrogen has two stable isotopes, 14 n and 15 n, whose relative abundances in nature are approximately 99.64% and 0.35%, respectively. 14n is the more abundant of the two, comprising 99.63%. The mass defect for the nucleus of helium is 0.0303 amu. Nitrogen. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT Characteristics of the Atmosphere PowerPoint Presentation, free Nitrogen In The Atmosphere Is 99.63 If average mass of nitrogen is 14.0067, calculate the relative abundance of both isotopes (14 n and 15 n respectively). 14n is the more abundant of the two, comprising 99.63%. Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). Nitrogen in the atmosphere is 99.63% 'n (14.00307 amu) and 0.37% 'n (15.00011 amu). Nitrogen. Nitrogen In The Atmosphere Is 99.63.
From www.slideserve.com
PPT KS4 Changes to the Earth and atmosphere PowerPoint Presentation Nitrogen In The Atmosphere Is 99.63 Nitrogen has two stable isotopes, 14n and 15n (atomic masses of 14 and 15, respectively). The mass defect for the nucleus of helium is 0.0303 amu. The binding energy per nucleon in m ev is. 14n is the more abundant of the two, comprising 99.63%. Atomic mass of nitrogen = 14.0067 amu. Isotopes can be defined as two or more. Nitrogen In The Atmosphere Is 99.63.