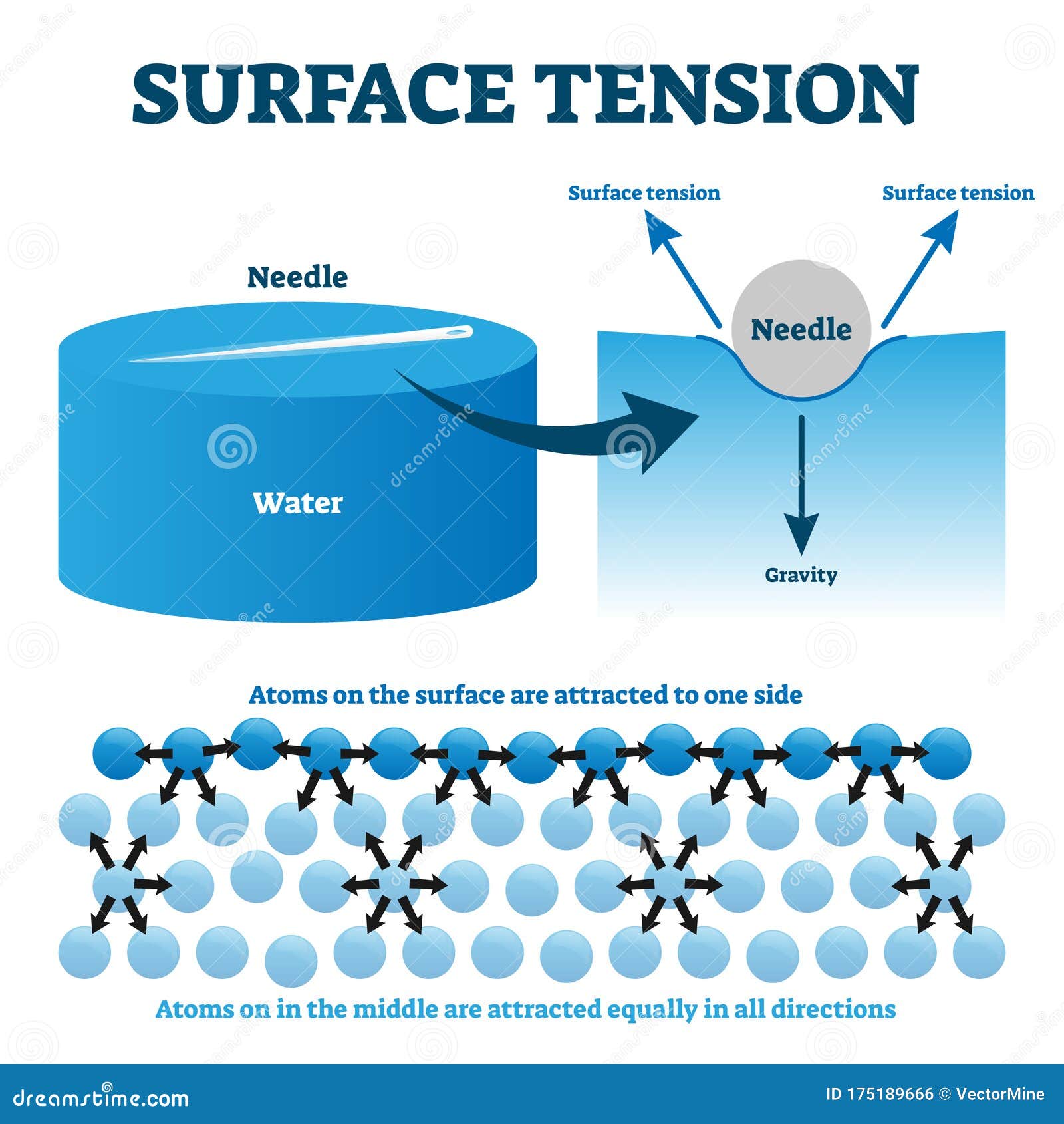
Surface Tension And Imfs . The stronger the intermolecular interactions, the. Molecules at the surface are less stable because they are. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Surface tension is the energy required to increase the surface area of a liquid by a given amount. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. In this article, you will learn about the effects of intermolecular forces (imfs). Liquids with strong intermolecular forces have. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which.
from www.dreamstime.com
Liquids with strong intermolecular forces have. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. Molecules at the surface are less stable because they are. The stronger the intermolecular interactions, the. In this article, you will learn about the effects of intermolecular forces (imfs). The surface tension of a liquid is a measure of the elastic force in the liquid's surface.
Surface Tension Explanation Vector Illustration Diagram Stock Vector
Surface Tension And Imfs Surface tension is the energy required to increase the surface area of a liquid by a given amount. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. Liquids with strong intermolecular forces have. In this article, you will learn about the effects of intermolecular forces (imfs). Molecules at the surface are less stable because they are. The surface tension of a liquid is a measure of the elastic force in the liquid's surface.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Surface Tension And Imfs Molecules at the surface are less stable because they are. In this article, you will learn about the effects of intermolecular forces (imfs). Surface tension is the amount of energy required to stretch or increase the surface of a liquid. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. The energy needed. Surface Tension And Imfs.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension And Imfs Liquids with strong intermolecular forces have. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. Surface tension is the energy required to increase. Surface Tension And Imfs.
From slideplayer.com
Liquids, Solids, and Intermolecular Forces ppt download Surface Tension And Imfs Molecules at the surface are less stable because they are. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. The core principle is that the stronger the imfs in the sample of. Surface Tension And Imfs.
From www.slideserve.com
PPT Covalent Compounds PowerPoint Presentation ID2170124 Surface Tension And Imfs Molecules at the surface are less stable because they are. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Surface tension is the energy required to increase the surface area of a liquid by a given amount. You will understand how intermolecular forces influence the physical properties of matter, such as melting. Surface Tension And Imfs.
From slideplayer.com
PHASES OF MATTER AND ENERGY ppt download Surface Tension And Imfs The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the. In this article, you will learn about the effects of. Surface Tension And Imfs.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension And Imfs Molecules at the surface are less stable because they are. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. Liquids with strong intermolecular forces have. The stronger the intermolecular interactions, the. The energy needed to. Surface Tension And Imfs.
From slideplayer.com
Intermolecular Forces ppt download Surface Tension And Imfs Liquids with strong intermolecular forces have. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. Molecules at the surface are less stable because they are. In this article, you. Surface Tension And Imfs.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID1598169 Surface Tension And Imfs The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. The stronger the intermolecular interactions, the. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Liquids with strong intermolecular forces have. Surface tension is the energy. Surface Tension And Imfs.
From slideplayer.com
IMFs in Action, and Vaporization and Vapor Pressure ppt download Surface Tension And Imfs The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. Molecules at the surface are less stable because they are. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. Surface tension is the. Surface Tension And Imfs.
From slideplayer.com
Intermolecular Forces ppt download Surface Tension And Imfs In this article, you will learn about the effects of intermolecular forces (imfs). Liquids with strong intermolecular forces have. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the amount of. Surface Tension And Imfs.
From slideplayer.com
Chapter 11 ( ) Intermolecular Forces, Liquids and Solids ppt download Surface Tension And Imfs The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. The stronger the. Surface Tension And Imfs.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement Surface Tension And Imfs In this article, you will learn about the effects of intermolecular forces (imfs). Molecules at the surface are less stable because they are. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. Liquids with strong intermolecular forces have. The surface tension of a liquid is a measure of the. Surface Tension And Imfs.
From slideplayer.com
Intermolecular Forces What holds everything together (Chapter 14 Surface Tension And Imfs Molecules at the surface are less stable because they are. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. Liquids with strong intermolecular forces have. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. In. Surface Tension And Imfs.
From www.youtube.com
Surface Tension & IMFs YouTube Surface Tension And Imfs Liquids with strong intermolecular forces have. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. The stronger the intermolecular interactions, the. In this article, you will. Surface Tension And Imfs.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Surface Tension And Imfs Liquids with strong intermolecular forces have. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the. You will understand how intermolecular forces influence the physical properties. Surface Tension And Imfs.
From slideplayer.com
Liquids and Solids. ppt download Surface Tension And Imfs Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Molecules at the surface are less stable because they are. You will understand how intermolecular forces influence the physical properties of matter, such as melting. Surface Tension And Imfs.
From www.learnatnoon.com
What is Surface Tension in Physics? Surface Tension And Imfs In this article, you will learn about the effects of intermolecular forces (imfs). The stronger the intermolecular interactions, the. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. Surface tension is the energy required to increase the surface area of a liquid by. Surface Tension And Imfs.
From wisc.pb.unizin.org
Explaining Solubility and Surface Tension through IMFs (M10Q4) UW Surface Tension And Imfs The stronger the intermolecular interactions, the. Liquids with strong intermolecular forces have. In this article, you will learn about the effects of intermolecular forces (imfs). Molecules at the surface are less stable because they are. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. Surface tension is the energy. Surface Tension And Imfs.
From wisc.pb.unizin.org
Explaining Solubility and Surface Tension through IMFs (M10Q4) UW Surface Tension And Imfs Surface tension is the energy required to increase the surface area of a liquid by a given amount. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Molecules at the surface are less stable because they are. Surface tension is the amount of energy required to stretch or increase the surface of. Surface Tension And Imfs.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension And Imfs The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. The stronger the intermolecular interactions, the. The surface tension of a liquid is a. Surface Tension And Imfs.
From slideplayer.com
Chapter 11 Intermolecular Forces, Liquids, and Solids ppt download Surface Tension And Imfs In this article, you will learn about the effects of intermolecular forces (imfs). Liquids with strong intermolecular forces have. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules. Surface Tension And Imfs.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Surface Tension And Imfs You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The energy needed to increase. Surface Tension And Imfs.
From www.youtube.com
SURFACE TENSION & INTERFACIAL PHENOMENON PART1 INTERFACE TYPES Surface Tension And Imfs The surface tension of a liquid is a measure of the elastic force in the liquid's surface. The stronger the intermolecular interactions, the. Molecules at the surface are less stable because they are. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. Surface. Surface Tension And Imfs.
From chem.libretexts.org
16.2 The Liquid State Chemistry LibreTexts Surface Tension And Imfs Surface tension is the energy required to increase the surface area of a liquid by a given amount. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. You will understand how intermolecular forces influence the physical properties of matter, such as melting point,. Surface Tension And Imfs.
From scienceinfo.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences Surface Tension And Imfs You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. In this article, you will learn about the effects of intermolecular forces (imfs). The energy needed to increase a liquid’s. Surface Tension And Imfs.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension And Imfs Surface tension is the energy required to increase the surface area of a liquid by a given amount. Molecules at the surface are less stable because they are. The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Liquids with strong intermolecular forces have. The energy needed to increase a liquid’s surface area. Surface Tension And Imfs.
From wisc.pb.unizin.org
M10Q4 Explaining Solubility and Surface Tension through IMFs Chem Surface Tension And Imfs Molecules at the surface are less stable because they are. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. You will understand how intermolecular forces. Surface Tension And Imfs.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Surface Tension And Imfs Surface tension is the energy required to increase the surface area of a liquid by a given amount. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. The stronger the intermolecular interactions, the. Molecules at the surface are less stable because they are.. Surface Tension And Imfs.
From www.youtube.com
Difference between surface and interface surface tension and Surface Tension And Imfs The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. The surface tension of a liquid is a measure of the elastic force in. Surface Tension And Imfs.
From slideplayer.com
Intermolecular Forces & Properties ppt download Surface Tension And Imfs Liquids with strong intermolecular forces have. The energy needed to increase a liquid’s surface area by a unit amount is called its surface tension, which is often measured in joules per meter. The stronger the intermolecular interactions, the. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the. Surface Tension And Imfs.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Surface Tension And Imfs The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The energy needed to increase a liquid’s surface. Surface Tension And Imfs.
From printablelibigergz.z21.web.core.windows.net
Viscosity Vs Surface Tension Surface Tension And Imfs The surface tension of a liquid is a measure of the elastic force in the liquid's surface. Molecules at the surface are less stable because they are. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The core principle is that the stronger the imfs in the sample of molecules, the. Surface Tension And Imfs.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Surface Tension And Imfs Liquids with strong intermolecular forces have. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. You will understand how intermolecular forces influence the physical properties of matter, such as melting point, viscosity, and surface tension. The energy needed to increase a liquid’s surface area by a unit amount is. Surface Tension And Imfs.
From slideplayer.com
Intermolecular Forces ppt download Surface Tension And Imfs Surface tension is the energy required to increase the surface area of a liquid by a given amount. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. Molecules at the surface are less stable because they are. Liquids with strong intermolecular forces have. You will understand how intermolecular forces. Surface Tension And Imfs.
From www.esaral.com
Mind Maps for Fluid Surface Tension Revision Class 11, JEE, NEET Surface Tension And Imfs Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the amount of energy required to stretch or increase the surface of a liquid. The core principle is that the stronger the imfs in the sample of molecules, the more strongly they interact, which. Molecules at the surface are. Surface Tension And Imfs.