Titration Calculations Hsc Questions . The equation for the titration reaction in step 2 is shown below: Find the moles used of the known solution (moles = conc. 4 steps to success with titration calculations 1. Calculate the initial number of moles of hcl present before the reaction in step 1. 1) evaluate the suitability of substance “x” as a. 2hcl + na2co3 → 2nacl + h2o + co2 in one. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Areas of titration questions with sample answers. A group of students conducted a series of titrations using the following steps: Washed burette with distilled water and a small quantity of acid before filling.
from studylib.net
Calculate the initial number of moles of hcl present before the reaction in step 1. Areas of titration questions with sample answers. 1) evaluate the suitability of substance “x” as a. Washed burette with distilled water and a small quantity of acid before filling. A group of students conducted a series of titrations using the following steps: 2hcl + na2co3 → 2nacl + h2o + co2 in one. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Find the moles used of the known solution (moles = conc. The equation for the titration reaction in step 2 is shown below: 4 steps to success with titration calculations 1.
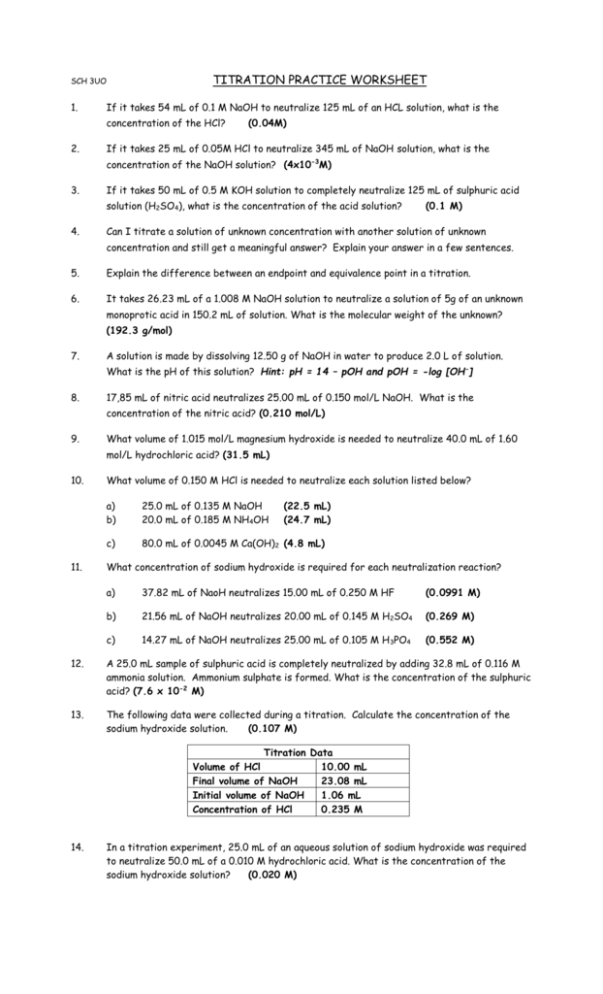
Titration Practice Worksheet
Titration Calculations Hsc Questions The equation for the titration reaction in step 2 is shown below: Washed burette with distilled water and a small quantity of acid before filling. A group of students conducted a series of titrations using the following steps: Areas of titration questions with sample answers. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Calculate the initial number of moles of hcl present before the reaction in step 1. Find the moles used of the known solution (moles = conc. 1) evaluate the suitability of substance “x” as a. The equation for the titration reaction in step 2 is shown below: 4 steps to success with titration calculations 1. 2hcl + na2co3 → 2nacl + h2o + co2 in one.
From www.reddit.com
Titration Calculations r/GCSE Titration Calculations Hsc Questions 1) evaluate the suitability of substance “x” as a. 4 steps to success with titration calculations 1. 2hcl + na2co3 → 2nacl + h2o + co2 in one. Calculate the initial number of moles of hcl present before the reaction in step 1. Areas of titration questions with sample answers. Washed burette with distilled water and a small quantity of. Titration Calculations Hsc Questions.
From www.revisely.co.uk
ALevel AQA Chemistry Questions pH Curves, Titrations and Indicators Titration Calculations Hsc Questions Washed burette with distilled water and a small quantity of acid before filling. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Calculate the initial number of moles of hcl present before the reaction in step 1. 4 steps to success with titration calculations 1. 2hcl + na2co3 → 2nacl. Titration Calculations Hsc Questions.
From www.vrogue.co
Titration Calculations And Questions Worksheet Teachi vrogue.co Titration Calculations Hsc Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 4 steps to success with titration calculations 1. 2hcl + na2co3 → 2nacl + h2o + co2 in one. Washed burette with distilled water and a small quantity of acid before filling. A group of students conducted a series of titrations. Titration Calculations Hsc Questions.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titration Calculations Hsc Questions The equation for the titration reaction in step 2 is shown below: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Calculate the initial number of moles of hcl present before the reaction in step 1. Find the moles used of the known solution (moles = conc. 2hcl + na2co3. Titration Calculations Hsc Questions.
From studylib.net
Titration Practice Worksheet Titration Calculations Hsc Questions Areas of titration questions with sample answers. Calculate the initial number of moles of hcl present before the reaction in step 1. The equation for the titration reaction in step 2 is shown below: Washed burette with distilled water and a small quantity of acid before filling. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water. Titration Calculations Hsc Questions.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Calculations Hsc Questions A group of students conducted a series of titrations using the following steps: Areas of titration questions with sample answers. The equation for the titration reaction in step 2 is shown below: 2hcl + na2co3 → 2nacl + h2o + co2 in one. 1) evaluate the suitability of substance “x” as a. A 1.0000 gram sample of k2co3 (138.2055 g/mol). Titration Calculations Hsc Questions.
From www.thinkswap.com
HSC Chemistry Practical Assessment Titration and Ion Identification Titration Calculations Hsc Questions Calculate the initial number of moles of hcl present before the reaction in step 1. A group of students conducted a series of titrations using the following steps: 4 steps to success with titration calculations 1. Areas of titration questions with sample answers. The equation for the titration reaction in step 2 is shown below: 2hcl + na2co3 → 2nacl. Titration Calculations Hsc Questions.
From edzion.com
HSC Titration Questions Edzion Titration Calculations Hsc Questions 4 steps to success with titration calculations 1. A group of students conducted a series of titrations using the following steps: Washed burette with distilled water and a small quantity of acid before filling. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 2hcl + na2co3 → 2nacl + h2o. Titration Calculations Hsc Questions.
From scienceready.com.au
Titration Calculation Examples and Solutions HSC Chemistry Science Titration Calculations Hsc Questions Areas of titration questions with sample answers. The equation for the titration reaction in step 2 is shown below: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Calculate the initial number of moles of hcl present before the reaction in step 1. 2hcl + na2co3 → 2nacl + h2o. Titration Calculations Hsc Questions.
From www.scribd.com
Titrations Calculations Questions PDF Sodium Hydroxide Titration Calculations Hsc Questions Calculate the initial number of moles of hcl present before the reaction in step 1. Areas of titration questions with sample answers. 1) evaluate the suitability of substance “x” as a. Find the moles used of the known solution (moles = conc. 2hcl + na2co3 → 2nacl + h2o + co2 in one. 4 steps to success with titration calculations. Titration Calculations Hsc Questions.
From blogs.glowscotland.org.uk
Acid & Alkali Revision (incl. Titration Calculation) National 5 Titration Calculations Hsc Questions 1) evaluate the suitability of substance “x” as a. Areas of titration questions with sample answers. The equation for the titration reaction in step 2 is shown below: Find the moles used of the known solution (moles = conc. Calculate the initial number of moles of hcl present before the reaction in step 1. Washed burette with distilled water and. Titration Calculations Hsc Questions.
From www.youtube.com
28 TITRATION 1 Experiment , Calculation HSC Chemistry YouTube Titration Calculations Hsc Questions 1) evaluate the suitability of substance “x” as a. Areas of titration questions with sample answers. Washed burette with distilled water and a small quantity of acid before filling. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. The equation for the titration reaction in step 2 is shown below:. Titration Calculations Hsc Questions.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse Titration Calculations Hsc Questions 2hcl + na2co3 → 2nacl + h2o + co2 in one. Find the moles used of the known solution (moles = conc. A group of students conducted a series of titrations using the following steps: 1) evaluate the suitability of substance “x” as a. Washed burette with distilled water and a small quantity of acid before filling. Areas of titration. Titration Calculations Hsc Questions.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Titration Calculations Hsc Questions The equation for the titration reaction in step 2 is shown below: Find the moles used of the known solution (moles = conc. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 2hcl + na2co3 → 2nacl + h2o + co2 in one. 4 steps to success with titration calculations. Titration Calculations Hsc Questions.
From www.youtube.com
TITRATION EXAM QUESTION for A LEVEL CHEMISTRY YouTube Titration Calculations Hsc Questions Calculate the initial number of moles of hcl present before the reaction in step 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. A group of students conducted a series of titrations using the following steps: The equation for the titration reaction in step 2 is shown below: 1). Titration Calculations Hsc Questions.
From www.studocu.com
Acids and Bases Titration Calculations Question 1 HSC 2003 25 of 0 Titration Calculations Hsc Questions 4 steps to success with titration calculations 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Find the moles used of the known solution (moles = conc. Washed burette with distilled water and a small quantity of acid before filling. Areas of titration questions with sample answers. Calculate the. Titration Calculations Hsc Questions.
From edzion.com
HSC Titration Questions Edzion Titration Calculations Hsc Questions 4 steps to success with titration calculations 1. Calculate the initial number of moles of hcl present before the reaction in step 1. 1) evaluate the suitability of substance “x” as a. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. A group of students conducted a series of titrations. Titration Calculations Hsc Questions.
From slideplayer.com
Acid Base Titrations Lesson ppt download Titration Calculations Hsc Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. A group of students conducted a series of titrations using the following steps: The equation for the titration reaction in step 2 is shown below: Calculate the initial number of moles of hcl present before the reaction in step 1. Washed. Titration Calculations Hsc Questions.
From www.tes.com
A level Chemistry Titration Calculations Teaching Resources Titration Calculations Hsc Questions A group of students conducted a series of titrations using the following steps: Areas of titration questions with sample answers. 1) evaluate the suitability of substance “x” as a. Washed burette with distilled water and a small quantity of acid before filling. Find the moles used of the known solution (moles = conc. Calculate the initial number of moles of. Titration Calculations Hsc Questions.
From www.coursehero.com
[Solved] Chemistry questions on a AcidBase Titration calculations Titration Calculations Hsc Questions 1) evaluate the suitability of substance “x” as a. 4 steps to success with titration calculations 1. A group of students conducted a series of titrations using the following steps: The equation for the titration reaction in step 2 is shown below: 2hcl + na2co3 → 2nacl + h2o + co2 in one. A 1.0000 gram sample of k2co3 (138.2055. Titration Calculations Hsc Questions.
From tukioka-clinic.com
️ Acid base titration problems with answers pdf. Eleventh grade Lesson Titration Calculations Hsc Questions 2hcl + na2co3 → 2nacl + h2o + co2 in one. Washed burette with distilled water and a small quantity of acid before filling. 4 steps to success with titration calculations 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Areas of titration questions with sample answers. 1) evaluate. Titration Calculations Hsc Questions.
From www.youtube.com
Back Titration Calculations NESA HSC Chemistry ThinkTank Education Titration Calculations Hsc Questions Washed burette with distilled water and a small quantity of acid before filling. The equation for the titration reaction in step 2 is shown below: Calculate the initial number of moles of hcl present before the reaction in step 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Areas. Titration Calculations Hsc Questions.
From www.youtube.com
A Level, IB, HSC, AP chemistry Backward titration (calculations Titration Calculations Hsc Questions Find the moles used of the known solution (moles = conc. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 4 steps to success with titration calculations 1. A group of students conducted a series of titrations using the following steps: Washed burette with distilled water and a small quantity. Titration Calculations Hsc Questions.
From www.youtube.com
Titration Calculations GCSE Science grade 7, 8 and 9 Booster Titration Calculations Hsc Questions Find the moles used of the known solution (moles = conc. 4 steps to success with titration calculations 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 2hcl + na2co3 → 2nacl + h2o + co2 in one. Washed burette with distilled water and a small quantity of acid. Titration Calculations Hsc Questions.
From www.studocu.com
Titration Calculations TITRATION CALCULATIONS WORKSHEET A 50 ml Titration Calculations Hsc Questions The equation for the titration reaction in step 2 is shown below: Washed burette with distilled water and a small quantity of acid before filling. 2hcl + na2co3 → 2nacl + h2o + co2 in one. Find the moles used of the known solution (moles = conc. A group of students conducted a series of titrations using the following steps:. Titration Calculations Hsc Questions.
From www.youtube.com
Titration calculations (CSEC Chemistry, January 2017, paper 2, question Titration Calculations Hsc Questions 2hcl + na2co3 → 2nacl + h2o + co2 in one. Washed burette with distilled water and a small quantity of acid before filling. Calculate the initial number of moles of hcl present before the reaction in step 1. 1) evaluate the suitability of substance “x” as a. The equation for the titration reaction in step 2 is shown below:. Titration Calculations Hsc Questions.
From edzion.com
HSC Titration Questions Edzion Titration Calculations Hsc Questions A group of students conducted a series of titrations using the following steps: 2hcl + na2co3 → 2nacl + h2o + co2 in one. The equation for the titration reaction in step 2 is shown below: 1) evaluate the suitability of substance “x” as a. Areas of titration questions with sample answers. Calculate the initial number of moles of hcl. Titration Calculations Hsc Questions.
From www.youtube.com
Acids and Bases Back Titration Calculation Exam Question|A Level Titration Calculations Hsc Questions 2hcl + na2co3 → 2nacl + h2o + co2 in one. Calculate the initial number of moles of hcl present before the reaction in step 1. 4 steps to success with titration calculations 1. Find the moles used of the known solution (moles = conc. Areas of titration questions with sample answers. Washed burette with distilled water and a small. Titration Calculations Hsc Questions.
From www.youtube.com
CSEC Chemistry Titrations and calculations YouTube Titration Calculations Hsc Questions Washed burette with distilled water and a small quantity of acid before filling. Find the moles used of the known solution (moles = conc. The equation for the titration reaction in step 2 is shown below: 1) evaluate the suitability of substance “x” as a. A group of students conducted a series of titrations using the following steps: 4 steps. Titration Calculations Hsc Questions.
From curriculum-press.co.uk
How to Answer Questions on Titration Calculations Curriculum Press Titration Calculations Hsc Questions Washed burette with distilled water and a small quantity of acid before filling. The equation for the titration reaction in step 2 is shown below: Find the moles used of the known solution (moles = conc. A group of students conducted a series of titrations using the following steps: 2hcl + na2co3 → 2nacl + h2o + co2 in one.. Titration Calculations Hsc Questions.
From www.chegg.com
Solved To review how titration calculations work, here's a Titration Calculations Hsc Questions A group of students conducted a series of titrations using the following steps: Washed burette with distilled water and a small quantity of acid before filling. 2hcl + na2co3 → 2nacl + h2o + co2 in one. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. The equation for the. Titration Calculations Hsc Questions.
From fyoeipkfz.blob.core.windows.net
Titration Calculations Hsc at Alice Short blog Titration Calculations Hsc Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Areas of titration questions with sample answers. The equation for the titration reaction in step 2 is shown below: Find the moles used of the known solution (moles = conc. 1) evaluate the suitability of substance “x” as a. Washed burette. Titration Calculations Hsc Questions.
From www.youtube.com
Titration Calculations YouTube Titration Calculations Hsc Questions A group of students conducted a series of titrations using the following steps: 4 steps to success with titration calculations 1. A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. Find the moles used of the known solution (moles = conc. Washed burette with distilled water and a small quantity. Titration Calculations Hsc Questions.
From classburns.z4.web.core.windows.net
Titration Calculations Practice Questions Titration Calculations Hsc Questions Find the moles used of the known solution (moles = conc. 1) evaluate the suitability of substance “x” as a. 2hcl + na2co3 → 2nacl + h2o + co2 in one. A group of students conducted a series of titrations using the following steps: A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0. Titration Calculations Hsc Questions.
From www.youtube.com
Back Titration Calculations // HSC Chemistry YouTube Titration Calculations Hsc Questions A 1.0000 gram sample of k2co3 (138.2055 g/mol) is dissolved in enough water to make 250.0 ml of solution. 1) evaluate the suitability of substance “x” as a. Washed burette with distilled water and a small quantity of acid before filling. Calculate the initial number of moles of hcl present before the reaction in step 1. 4 steps to success. Titration Calculations Hsc Questions.