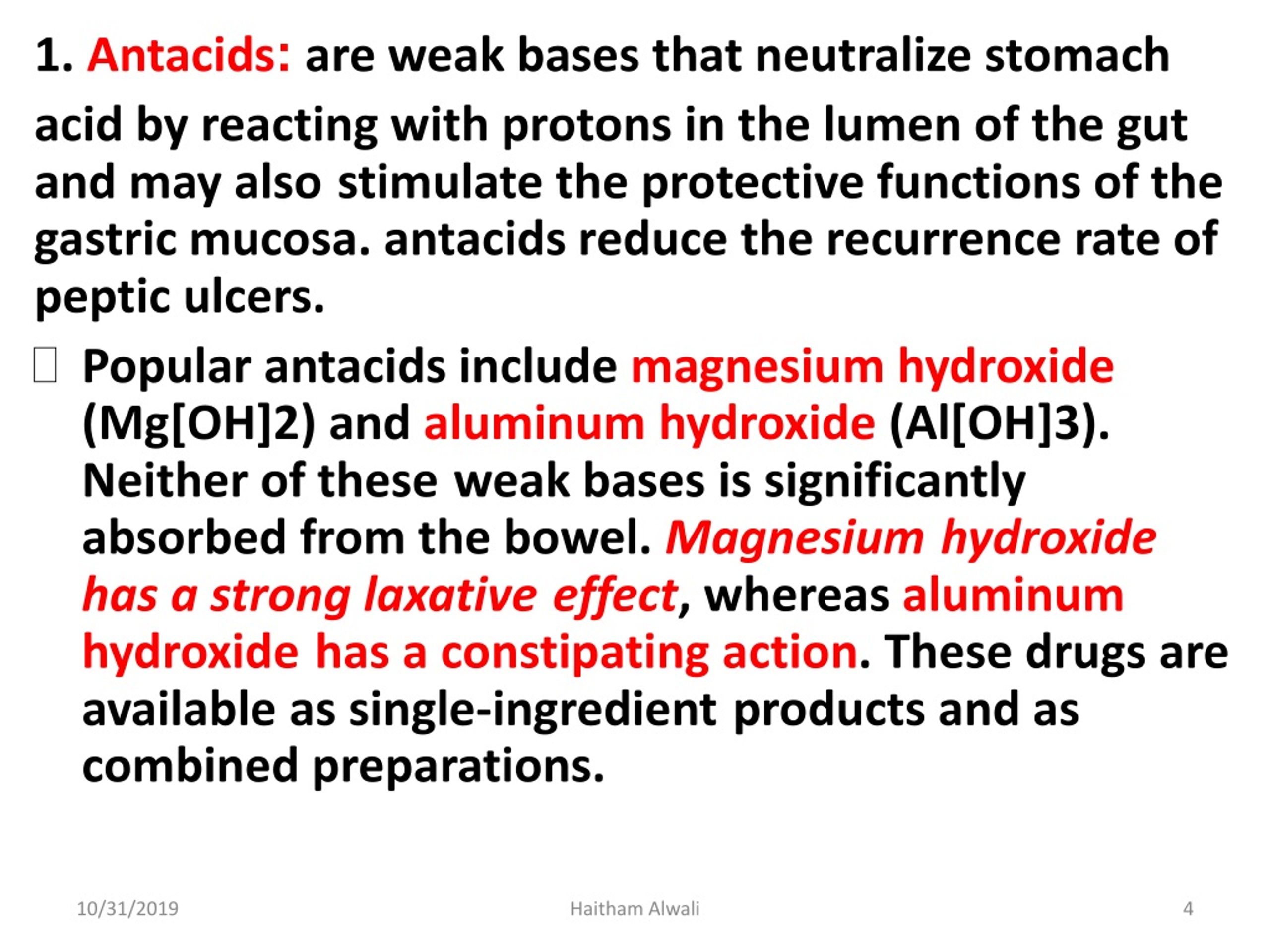
Antacids Contain Weak Base . Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Antacids are weak bases that act by neutralizing gastric acid (fig. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Raising the ph of the stomach contents results in suppression of the. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Perform a back titration experiment to.
from www.slideserve.com
Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Perform a back titration experiment to. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Raising the ph of the stomach contents results in suppression of the. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Antacids are weak bases that act by neutralizing gastric acid (fig.
PPT Drugs used in GIT disorders PowerPoint Presentation, free
Antacids Contain Weak Base Perform a back titration experiment to. Antacids are weak bases that act by neutralizing gastric acid (fig. The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Raising the ph of the stomach contents results in suppression of the. Perform a back titration experiment to. Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Antacids are weak bases because it contains magnesium hydroxide which is weak in nature
From www.studocu.com
Module 11 Chapter 17 Antacids o Weak bases that react with Antacids Contain Weak Base Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Antacids are weak bases that act by neutralizing gastric acid (fig. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Perform a. Antacids Contain Weak Base.
From slideplayer.com
Acid secretion and antacids ppt download Antacids Contain Weak Base Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Antacids are weak bases that act by neutralizing gastric acid (fig. Perform a back titration experiment to. The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Raising the ph of the stomach. Antacids Contain Weak Base.
From slideplayer.com
Antacids Assessment Statement ppt download Antacids Contain Weak Base Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Raising the ph of the stomach contents results in suppression of the. Antacids are weak bases that act by neutralizing gastric acid (fig. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Amines, which are organic analogues of ammonia,. Antacids Contain Weak Base.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Antacids Contain Weak Base Antacids are weak bases that act by neutralizing gastric acid (fig. Perform a back titration experiment to. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). The antacids. Antacids Contain Weak Base.
From slideplayer.com
Properties of Acids and Bases ppt download Antacids Contain Weak Base Perform a back titration experiment to. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Raising the ph of the stomach contents results in suppression of the. Antacids are weak bases that act by neutralizing gastric acid (fig. Learn how to use titration to measure the number of moles of h+ neutralized per gram of. Antacids Contain Weak Base.
From www.slideserve.com
PPT Acid, Bases, and Salts PowerPoint Presentation, free download Antacids Contain Weak Base The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Perform a back titration experiment to. Amines, which are organic analogues. Antacids Contain Weak Base.
From www.slideserve.com
PPT Weak acids and bases Salt of weak acid and bases buffer Lecture 9 Antacids Contain Weak Base Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Antacids are weak bases that act by neutralizing gastric acid (fig. Raising the ph of the. Antacids Contain Weak Base.
From slidetodoc.com
Gastrointestinal Pharmacology Antacids Peptic ulcer therapy Antiemetics Antacids Contain Weak Base Perform a back titration experiment to. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Learn about different antacids and how they. Antacids Contain Weak Base.
From www.slideserve.com
PPT Solutions of acids & bases PowerPoint Presentation, free download Antacids Contain Weak Base Antacids are weak bases that act by neutralizing gastric acid (fig. The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Learn how to use. Antacids Contain Weak Base.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Antacids Contain Weak Base Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Raising the ph of the stomach contents results in suppression of the. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). The antacids act by. Antacids Contain Weak Base.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Antacids Contain Weak Base The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Perform a back titration experiment to. Antacids are weak bases that act by neutralizing gastric acid (fig. Antacids are weak bases because it contains magnesium hydroxide. Antacids Contain Weak Base.
From arturo-kmeyer.blogspot.com
Name 2 Compounds Which Are Used as Antacids Antacids Contain Weak Base Raising the ph of the stomach contents results in suppression of the. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). The antacids act by neutralizing the acid in. Antacids Contain Weak Base.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Antacids Contain Weak Base Raising the ph of the stomach contents results in suppression of the. Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn.. Antacids Contain Weak Base.
From www.slideserve.com
PPT Chapter 8 Acids and Bases PowerPoint Presentation, free download Antacids Contain Weak Base Perform a back titration experiment to. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Antacids are weak bases that act by neutralizing gastric acid (fig. Raising the. Antacids Contain Weak Base.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID3824036 Antacids Contain Weak Base Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Perform a back titration experiment to. Learn how antacids neutralize hydrochloric acid (hcl) in the. Antacids Contain Weak Base.
From ndclist.com
Label Regular Strength Antacid Mint Liquid Oral Indications, Usage Antacids Contain Weak Base Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Perform a back titration experiment to. The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak. Antacids Contain Weak Base.
From slideplayer.com
Chapter 15 D.4 pH regulation D.5 Antiviral Meds ppt download Antacids Contain Weak Base Perform a back titration experiment to. Antacids are weak bases that act by neutralizing gastric acid (fig. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Raising the ph of the stomach contents results in suppression of the. Learn how to use titration to measure the number of moles of h+ neutralized per gram. Antacids Contain Weak Base.
From www.slideserve.com
PPT Acids, Bases, and Salts PowerPoint Presentation, free download Antacids Contain Weak Base Raising the ph of the stomach contents results in suppression of the. Antacids are weak bases that act by neutralizing gastric acid (fig. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Learn how to use titration to measure the. Antacids Contain Weak Base.
From www.slideserve.com
PPT Chapter 5 Acids and Bases PowerPoint Presentation, free download Antacids Contain Weak Base Raising the ph of the stomach contents results in suppression of the. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). The antacids act by neutralizing the acid. Antacids Contain Weak Base.
From www.scribd.com
Comparitive Study of Commercial Antacids PDF Titration Chemistry Antacids Contain Weak Base Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Antacids are weak bases. Antacids Contain Weak Base.
From slideplayer.com
Antacids 2B This unit will introduce the chemistry needed to Antacids Contain Weak Base Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Antacids are weak bases that act by neutralizing gastric acid (fig. Raising the ph of the stomach contents results in suppression of the. Learn about different antacids. Antacids Contain Weak Base.
From www.chegg.com
Solved The antacids used in lab are all weak bases that work Antacids Contain Weak Base Raising the ph of the stomach contents results in suppression of the. Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Antacids are weak bases that act by neutralizing gastric acid (fig. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Perform a back titration. Antacids Contain Weak Base.
From mungfali.com
Weak Acids And Bases List Antacids Contain Weak Base The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Perform a back titration experiment to. Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Learn about different. Antacids Contain Weak Base.
From www.slideserve.com
PPT Antiemetics, antacids & GI motility drugs PowerPoint Antacids Contain Weak Base Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Raising the ph of the stomach contents results in suppression of the. Perform a back titration experiment to. Antacids. Antacids Contain Weak Base.
From www.slideserve.com
PPT Drugs used in GIT disorders PowerPoint Presentation, free Antacids Contain Weak Base Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Perform a back titration experiment to. Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Antacids are weak bases because it. Antacids Contain Weak Base.
From slideplayer.com
Click a hyperlink to view the corresponding slides. ppt download Antacids Contain Weak Base Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Perform a back titration experiment to. Antacids are. Antacids Contain Weak Base.
From www.slideserve.com
PPT The Acid Test PowerPoint Presentation, free download ID3938157 Antacids Contain Weak Base Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Raising the ph of the stomach contents results in suppression of the. Antacids are weak bases that act by neutralizing gastric acid (fig. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such. Antacids Contain Weak Base.
From slideplayer.com
Drugs acting on gastrointestinal tract ppt download Antacids Contain Weak Base Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Perform a back titration experiment to. Learn how to use titration to measure the number of moles of h+. Antacids Contain Weak Base.
From slideplayer.com
Chapter 15 D.4 pH regulation D.5 Antiviral Meds ppt download Antacids Contain Weak Base Perform a back titration experiment to. Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Raising the ph of the stomach contents results in suppression of the. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Amines, which are organic analogues of ammonia, are also. Antacids Contain Weak Base.
From www.slideserve.com
PPT The Acid Test PowerPoint Presentation, free download ID3938157 Antacids Contain Weak Base The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Learn how to use titration to measure the number of moles of h+ neutralized per gram of antacid. Amines, which are organic analogues of ammonia, are also. Antacids Contain Weak Base.
From www.numerade.com
SOLVED Many common antacid products contain CaCO3, which behaves as a Antacids Contain Weak Base Perform a back titration experiment to. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with. Antacids Contain Weak Base.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID1781850 Antacids Contain Weak Base Perform a back titration experiment to. Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Antacids are weak bases that act by neutralizing gastric acid (fig. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Raising the ph of the stomach contents results in suppression of the. The. Antacids Contain Weak Base.
From slideplayer.com
Chapter 15 D.4 pH regulation D.5 Antiviral Meds ppt download Antacids Contain Weak Base Perform a back titration experiment to. Antacids are weak bases because it contains magnesium hydroxide which is weak in nature Learn how antacids neutralize hydrochloric acid (hcl) in the stomach by reacting with weak bases. Raising the ph of the stomach contents results in suppression of the. The antacids act by neutralizing the acid in the stomach and by inhibiting. Antacids Contain Weak Base.
From www.slideserve.com
PPT Antacids PowerPoint Presentation ID1994845 Antacids Contain Weak Base The antacids act by neutralizing the acid in the stomach and by inhibiting pepsin, which is a proteolytic enzyme. Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Antacids are weak bases that act by neutralizing gastric acid (fig. Perform a back titration experiment to. Antacids are weak bases because it contains magnesium hydroxide. Antacids Contain Weak Base.
From www.chegg.com
Solved Find the weak base used in three commercial antacids. Antacids Contain Weak Base Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as s 2−). Learn about different antacids and how they work to neutralize stomach acid and relieve heartburn. Antacids are weak bases that act by neutralizing gastric acid (fig. Antacids are weak bases because it contains magnesium. Antacids Contain Weak Base.