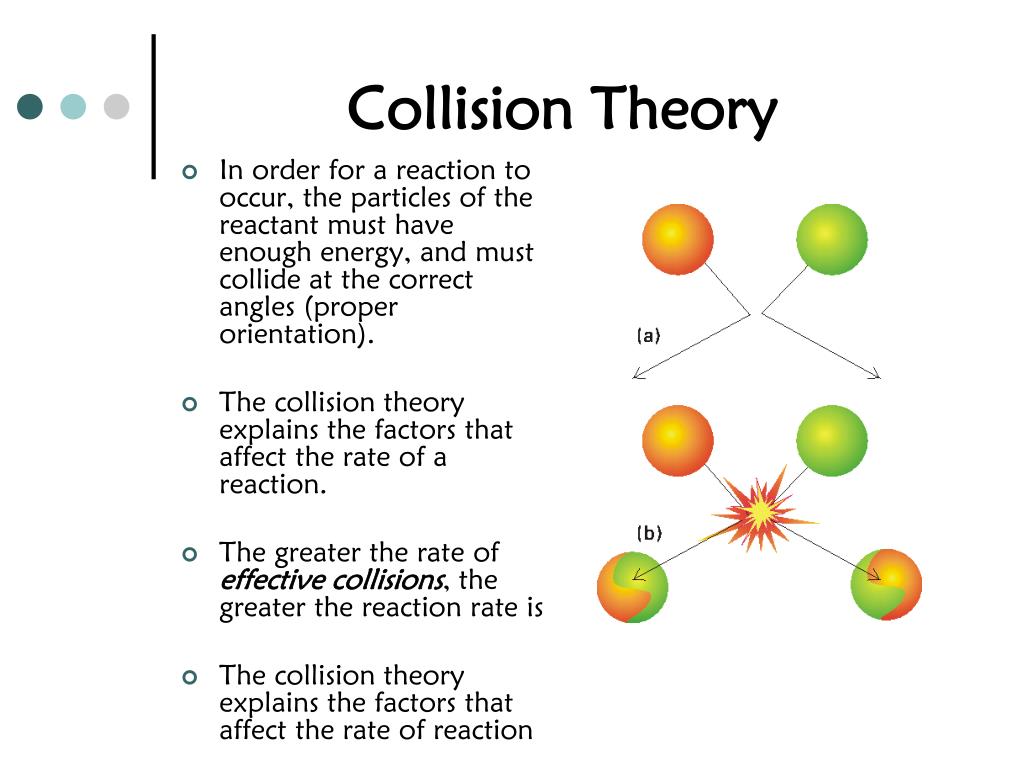
Effect Collision Of Molecules . By the end of this section, you will be able to: collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory explains why most reaction rates increase as concentrations increase. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. These successful changes are called successful collisions. Use the postulates of collision theory to explain the effects of physical state,. With an increase in the concentration of. the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls.
from www.slideserve.com
These successful changes are called successful collisions. collision theory explains why most reaction rates increase as concentrations increase. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Use the postulates of collision theory to explain the effects of physical state,. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. With an increase in the concentration of. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction.
PPT Collision Theory PowerPoint Presentation, free download ID3873529
Effect Collision Of Molecules By the end of this section, you will be able to: By the end of this section, you will be able to: These successful changes are called successful collisions. Use the postulates of collision theory to explain the effects of physical state,. collision theory explains why most reaction rates increase as concentrations increase. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. With an increase in the concentration of.
From www.slideshare.net
Rate of reaction chemical (condensed) Effect Collision Of Molecules the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. By the end of this section, you will be able to: These successful changes are called successful collisions. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With an. Effect Collision Of Molecules.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID1991748 Effect Collision Of Molecules increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. collision theory explains why most reaction rates increase as concentrations increase. collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. collision theory provides a simple but effective explanation for. Effect Collision Of Molecules.
From www.slideserve.com
PPT Collision of a Molecule with a Wall PowerPoint Presentation, free download ID5543370 Effect Collision Of Molecules With an increase in the concentration of. the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. collision theory explains why most reaction rates increase as concentrations increase. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. . Effect Collision Of Molecules.
From www.slideserve.com
PPT Chapter 10 Gases & the Atmosphere PowerPoint Presentation ID611285 Effect Collision Of Molecules By the end of this section, you will be able to: collision theory explains why most reaction rates increase as concentrations increase. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; collision theory provides a simple but effective. Effect Collision Of Molecules.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Effect Collision Of Molecules collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; These successful changes are called successful collisions. collision theory explains why. Effect Collision Of Molecules.
From thechemistrynotes.com
Collision Theory of Chemical Effect Collision Of Molecules With an increase in the concentration of. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. collision theory provides a. Effect Collision Of Molecules.
From byjus.com
Collision Theory Definition, Explanation, Activation energy, Arrhenius equation with Videos and Effect Collision Of Molecules By the end of this section, you will be able to: the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. Use the postulates of collision theory to explain the effects of physical state,. collision theory explains why most reaction rates increase as concentrations increase. These successful changes. Effect Collision Of Molecules.
From www.slideserve.com
PPT Collision of a Molecule with a Wall PowerPoint Presentation, free download ID5543370 Effect Collision Of Molecules Use the postulates of collision theory to explain the effects of physical state,. These successful changes are called successful collisions. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. collision theory. Effect Collision Of Molecules.
From www.researchgate.net
The illustration of the molecules/atoms in collision process. Download Scientific Diagram Effect Collision Of Molecules collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. Use the postulates of collision theory to explain the effects of physical state,. These successful changes are called successful collisions. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions. Effect Collision Of Molecules.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID3873529 Effect Collision Of Molecules By the end of this section, you will be able to: the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; These successful changes are called successful collisions. collision theory provides a simple but effective explanation for the effect of. Effect Collision Of Molecules.
From www.slideserve.com
PPT Chemical Reactions and Collision Theory PowerPoint Presentation, free download ID1833784 Effect Collision Of Molecules collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. By the end of this section, you will be able to: collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. the collision theory states that when suitable particles of the reactant. Effect Collision Of Molecules.
From saylordotorg.github.io
The Collision Model of Chemical Effect Collision Of Molecules collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. These successful changes are called successful collisions. By the end of this section, you will be able to: collision theory provides. Effect Collision Of Molecules.
From courses.lumenlearning.com
Collision Theory General Chemistry Effect Collision Of Molecules By the end of this section, you will be able to: collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. collision theory explains why most reaction rates increase as concentrations increase. Use the postulates of collision theory to explain the effects of physical state,. collision theory provides a. Effect Collision Of Molecules.
From www.tes.com
Collision Theory (Tempurature Effect And Molecular Theory) Handwritten IB Chemistry Effect Collision Of Molecules collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; the pressure exerted by a gas in a container results from. Effect Collision Of Molecules.
From www.slideserve.com
PPT Collision of a Molecule with a Wall PowerPoint Presentation, free download ID5543370 Effect Collision Of Molecules increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. These successful changes are called successful collisions. collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. By the end of this section, you will be able to: Use the postulates of. Effect Collision Of Molecules.
From www.youtube.com
Collision Theory and Reaction Rate // HSC Chemistry YouTube Effect Collision Of Molecules collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates. Effect Collision Of Molecules.
From www.slideserve.com
PPT Collision Theory & Reaction Mechanisms PowerPoint Presentation ID2263561 Effect Collision Of Molecules collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. collision theory explains why most reaction rates increase as concentrations increase. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. By the end of this section, you will be able to:. Effect Collision Of Molecules.
From www.youtube.com
Collisions of electrons with atoms (Quantum Phenomena 3) YouTube Effect Collision Of Molecules These successful changes are called successful collisions. collision theory explains why most reaction rates increase as concentrations increase. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions. Effect Collision Of Molecules.
From slideplayer.com
Chapter 17 Equilibrium Reversible Reactions. ppt download Effect Collision Of Molecules These successful changes are called successful collisions. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. By the end of this. Effect Collision Of Molecules.
From www.youtube.com
Molecular Collisions YouTube Effect Collision Of Molecules collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. collision theory explains why most reaction rates increase as concentrations increase. These successful changes are called successful collisions. the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. . Effect Collision Of Molecules.
From www.slideserve.com
PPT Collision of a Molecule with a Wall PowerPoint Presentation, free download ID5543370 Effect Collision Of Molecules collision theory explains why most reaction rates increase as concentrations increase. With an increase in the concentration of. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. By the end of this section, you will be able to: collision theory provides a simple but effective explanation for the effect. Effect Collision Of Molecules.
From www.slideshare.net
Chapter13 chemical Effect Collision Of Molecules collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. increasing. Effect Collision Of Molecules.
From slidetodoc.com
CHEM 3310 Chemical Collision Theory Transition State Effect Collision Of Molecules These successful changes are called successful collisions. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. collision theory provides a simple but effective explanation for the effect of many. Effect Collision Of Molecules.
From www.slideserve.com
PPT Collision of a Molecule with a Wall PowerPoint Presentation, free download ID5543370 Effect Collision Of Molecules These successful changes are called successful collisions. Use the postulates of collision theory to explain the effects of physical state,. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls.. Effect Collision Of Molecules.
From www.nagwa.com
Question Video Naming the Theory Describing How Chemical Reactions Occur after Particle Effect Collision Of Molecules collision theory explains why most reaction rates increase as concentrations increase. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates of collision theory to explain the effects of physical state,. These successful changes are called successful collisions. collision theory provides a simple but effective explanation. Effect Collision Of Molecules.
From www.researchgate.net
Consecutive collision of molecule í µí± with molecules í µí± 1 and í... Download Scientific Effect Collision Of Molecules increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; collision theory explains why most reaction rates increase as concentrations increase.. Effect Collision Of Molecules.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID1951310 Effect Collision Of Molecules the pressure exerted by a gas in a container results from collisions between the gas molecules and the container walls. With an increase in the concentration of. collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. collision theory provides a simple but effective explanation for the effect of. Effect Collision Of Molecules.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 Effect Collision Of Molecules collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. These successful changes are called successful collisions. collision theory explains why most reaction rates increase as concentrations increase. With an increase in. Effect Collision Of Molecules.
From www.youtube.com
Molecular Collisions Animation YouTube Effect Collision Of Molecules collision theory explains why most reaction rates increase as concentrations increase. Use the postulates of collision theory to explain the effects of physical state,. These successful changes are called successful collisions. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. collision theory states that for a chemical reaction. Effect Collision Of Molecules.
From www.slideserve.com
PPT Ch. 12 Behavior of Gases PowerPoint Presentation, free download ID1913193 Effect Collision Of Molecules collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of. Use the postulates of collision theory to explain the effects of physical state,. These successful changes are called successful collisions. the pressure. Effect Collision Of Molecules.
From learningschoolbocadeaswb.z4.web.core.windows.net
Elastic Inelastic And Completely Collisions Effect Collision Of Molecules collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. With an increase in the concentration of. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. the pressure exerted by a gas in a container results from collisions between the. Effect Collision Of Molecules.
From www.slideserve.com
PPT Chemical CHAPTER 14 Part B Chemistry The Molecular Nature of Matter, 6 th Effect Collision Of Molecules collision theory explains why most reaction rates increase as concentrations increase. These successful changes are called successful collisions. collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. the pressure. Effect Collision Of Molecules.
From www.slideserve.com
PPT Chapter 18 Chemical Equilibrium PowerPoint Presentation, free download ID2089339 Effect Collision Of Molecules collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. By the end of this section, you will be able to: collision theory explains why most reaction rates increase as concentrations increase. the pressure exerted by a gas in a container results from collisions between the gas molecules and the. Effect Collision Of Molecules.
From www.pinterest.com
Collision Theory Easy Science Collision theory, Theories, Theory definition Effect Collision Of Molecules collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. With an increase in the concentration of. collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing. Effect Collision Of Molecules.
From www.researchgate.net
Consecutive collision of molecule with molecules and. The distance... Download Scientific Diagram Effect Collision Of Molecules collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. the collision theory states that when suitable particles of the reactant hit each other, only a certain percentage of the collisions cause any noticeable or significant chemical change; Use the postulates of collision theory to explain the effects of physical. Effect Collision Of Molecules.