How Long Does It Take For A Drug To Be Generic . The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Drug companies must submit an abbreviated new drug application (called anda) for approval to. A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. The fda approves generic drugs through a specific process. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Generic versions can be equivalents or authorized generics, which are exact. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. How are generic drugs approved?
from hubpages.com
Generic versions can be equivalents or authorized generics, which are exact. Drug companies must submit an abbreviated new drug application (called anda) for approval to. The fda approves generic drugs through a specific process. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. How are generic drugs approved? A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively.
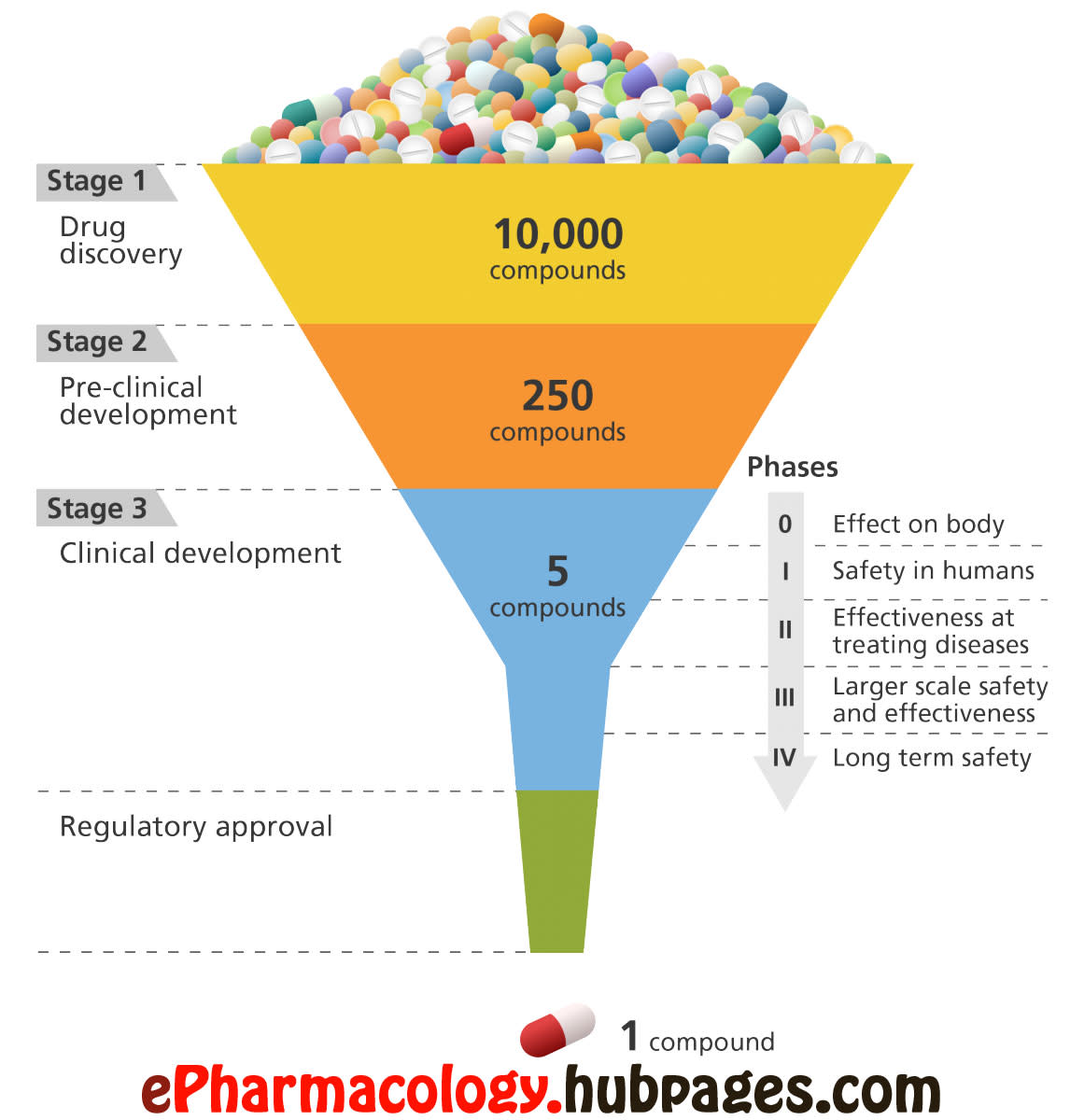
How are drugs developed and approved? The drug development process
How Long Does It Take For A Drug To Be Generic The fda approves generic drugs through a specific process. A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. The fda approves generic drugs through a specific process. Generic versions can be equivalents or authorized generics, which are exact. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. How are generic drugs approved? This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Drug companies must submit an abbreviated new drug application (called anda) for approval to.
From carolinacenterforrecovery.com
How Long Does Cocaine Stay in Your System? Urine, Blood, Saliva How Long Does It Take For A Drug To Be Generic Drug companies must submit an abbreviated new drug application (called anda) for approval to. How are generic drugs approved? A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. Generic drugs are approved only after a rigorous review by fda and after a set period of time. How Long Does It Take For A Drug To Be Generic.
From autosate.com
How Long Does It Take To Change Transmission Fluid? We talk all about How Long Does It Take For A Drug To Be Generic Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic versions can be equivalents or authorized generics, which are exact. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Generic drugs are approved only after a rigorous review by fda and. How Long Does It Take For A Drug To Be Generic.
From www.pgpf.org
How Much Does the United States Spend on Prescription Drugs Compared to How Long Does It Take For A Drug To Be Generic This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Generic versions can be equivalents or authorized generics, which are exact. How are generic drugs approved? Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been. How Long Does It Take For A Drug To Be Generic.
From trendofhealth.com
How Long Does It Take To Walk 2.5 Miles By Age And Gender? Trend Of How Long Does It Take For A Drug To Be Generic Generic versions can be equivalents or authorized generics, which are exact. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. The fda approves generic drugs through a specific process. How are generic drugs approved? A generic drug can be approved for a use that is not protected by patents. How Long Does It Take For A Drug To Be Generic.
From outdoorpositive.com
How Long Does It Take for a Bass to Digest Food? Outdoor Positive How Long Does It Take For A Drug To Be Generic The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Drug companies must submit an abbreviated new drug application (called anda) for approval to. How are generic. How Long Does It Take For A Drug To Be Generic.
From freebythesea.com
How Long Do Drugs Stay in Your System Free by the Sea How Long Does It Take For A Drug To Be Generic This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. The fda approves generic drugs through a specific process. Generic versions can be equivalents or authorized generics,. How Long Does It Take For A Drug To Be Generic.
From hxeljjwqk.blob.core.windows.net
How Long Does It Take For Carpet Grass To Grow at Cindy Kline blog How Long Does It Take For A Drug To Be Generic Drug companies must submit an abbreviated new drug application (called anda) for approval to. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Generic drugs are. How Long Does It Take For A Drug To Be Generic.
From www.lihpao.com
How Long Does It Take For Lasix To Work? Exploring the Speed of Lasix How Long Does It Take For A Drug To Be Generic Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted. How Long Does It Take For A Drug To Be Generic.
From techcult.com
How Long Does It Take for a Discord Account to Delete? TechCult How Long Does It Take For A Drug To Be Generic The fda approves generic drugs through a specific process. Generic versions can be equivalents or authorized generics, which are exact. Drug companies must submit an abbreviated new drug application (called anda) for approval to. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. How are generic. How Long Does It Take For A Drug To Be Generic.
From blogdigger.com
How Long Does It Take For Roblox To Respond? [2024] How Long Does It Take For A Drug To Be Generic A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. The generic drug approval process in japan in japan,. How Long Does It Take For A Drug To Be Generic.
From cilisos.my
Msian govt may control drug prices as private hospitals allegedly sell How Long Does It Take For A Drug To Be Generic A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. The fda approves generic drugs through a specific process. Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic versions can be equivalents or authorized generics, which are exact. The generic drug. How Long Does It Take For A Drug To Be Generic.
From www.sjrp.com
How Long Does Meth Stay In Your System? Hair, Saliva, Blood, Urine How Long Does It Take For A Drug To Be Generic The fda approves generic drugs through a specific process. A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. How are generic drugs approved? This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Drug companies must. How Long Does It Take For A Drug To Be Generic.
From healthmatch.io
HealthMatch Are Generic Drugs Just As Good As Branded Drugs? How Long Does It Take For A Drug To Be Generic A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. How are generic drugs approved? The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Generic drugs are approved only after a rigorous review. How Long Does It Take For A Drug To Be Generic.
From technologyrivers.com
How Long Does It Take To Develop An App? Technology Rivers How Long Does It Take For A Drug To Be Generic A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. The fda approves generic drugs through a specific process. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Generic versions can be equivalents or authorized generics,. How Long Does It Take For A Drug To Be Generic.
From www.debra.org.uk
How drugs are approved DEBRA UK The butterfly skin charity How Long Does It Take For A Drug To Be Generic The fda approves generic drugs through a specific process. Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic versions can be equivalents or authorized generics, which are exact. A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. This bioequivalence assessment. How Long Does It Take For A Drug To Be Generic.
From www.flow.ninja
How long does it take to learn flow How Long Does It Take For A Drug To Be Generic A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. How are generic drugs approved? This bioequivalence assessment helps. How Long Does It Take For A Drug To Be Generic.
From slsi.lk
slsi.lk how long for sulfatrim to work This antiretroviral drugs How Long Does It Take For A Drug To Be Generic Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. Generic versions can be equivalents or authorized generics, which are exact. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. How. How Long Does It Take For A Drug To Be Generic.
From www.goodrx.com
How Long Does It Take for Laxatives to Work? GoodRx How Long Does It Take For A Drug To Be Generic This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. How are generic drugs approved? Drug companies must submit an abbreviated new drug application (called anda) for. How Long Does It Take For A Drug To Be Generic.
From zeropestng.com
How long does it take for mosquito eggs to hatch How Long Does It Take For A Drug To Be Generic The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. The fda approves generic drugs through a specific process. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively.. How Long Does It Take For A Drug To Be Generic.
From ca.news.yahoo.com
Here's how long various drugs stay in your body How Long Does It Take For A Drug To Be Generic Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. How are generic drugs approved? The generic drug approval process in japan in japan, the pmda reviews generic. How Long Does It Take For A Drug To Be Generic.
From finance.yahoo.com
Here's how long different drugs stay in your system How Long Does It Take For A Drug To Be Generic Generic versions can be equivalents or authorized generics, which are exact. A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Drug companies must submit an abbreviated. How Long Does It Take For A Drug To Be Generic.
From healer.com
THC Dosage Chart & Guide How much THC should I take? How Long Does It Take For A Drug To Be Generic Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. This bioequivalence assessment helps to confirm the quality, efficacy,. How Long Does It Take For A Drug To Be Generic.
From www.sjrp.com
How Long Does Acid (LSD) Stay in Your System? SJRP Drug & Alcohol How Long Does It Take For A Drug To Be Generic Generic versions can be equivalents or authorized generics, which are exact. A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. The fda approves generic drugs through a specific process. Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic drugs are. How Long Does It Take For A Drug To Be Generic.
From www.homefortheharvest.com
How long does it take for a sunflower to grow? 🌻⏳ A timeline for your How Long Does It Take For A Drug To Be Generic A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Generic drugs are approved only after a rigorous review by fda and after a. How Long Does It Take For A Drug To Be Generic.
From livewell.com
How Long Does It Take For Insurance To Pay Out On A Stolen Vehicle How Long Does It Take For A Drug To Be Generic A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Generic versions can be equivalents or authorized generics, which are exact. How are generic. How Long Does It Take For A Drug To Be Generic.
From fabalabse.com
How long does it take to get approved for Venmo card? Leia aqui How How Long Does It Take For A Drug To Be Generic This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. The fda approves generic drugs through a specific process. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. A generic drug can be approved for a. How Long Does It Take For A Drug To Be Generic.
From hubpages.com
How are drugs developed and approved? The drug development process How Long Does It Take For A Drug To Be Generic Drug companies must submit an abbreviated new drug application (called anda) for approval to. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. How are generic drugs approved? A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove. How Long Does It Take For A Drug To Be Generic.
From contentatscale.ai
how long does it take to write a book How Long Does It Take For A Drug To Be Generic A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Drug companies must submit an abbreviated new drug application (called anda) for approval to. How are generic. How Long Does It Take For A Drug To Be Generic.
From tech.co
How Long Does It Take for a Hacker to Crack a Password? 🤔 How Long Does It Take For A Drug To Be Generic Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. The fda approves generic drugs through a specific process. The generic drug approval process in japan in japan,. How Long Does It Take For A Drug To Be Generic.
From blog.bankaletihad.com
How long does it take to transfer money? Bank al Etihad Jordan How Long Does It Take For A Drug To Be Generic The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has. How Long Does It Take For A Drug To Be Generic.
From piercingdepot.com
How Long Does It Take A Septum Piercing To Heal? PiercingDepot How Long Does It Take For A Drug To Be Generic How are generic drugs approved? Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Drug companies must submit. How Long Does It Take For A Drug To Be Generic.
From www.collidu.com
Generic Drugs Vs Branded Drugs PowerPoint and Google Slides Template How Long Does It Take For A Drug To Be Generic Generic drugs are approved only after a rigorous review by fda and after a set period of time that the brand product has been on the market exclusively. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. How are generic drugs approved? Drug companies must submit. How Long Does It Take For A Drug To Be Generic.
From www.aol.com
Here's how long various drugs stay in your body AOL News How Long Does It Take For A Drug To Be Generic How are generic drugs approved? Generic versions can be equivalents or authorized generics, which are exact. A generic drug can be approved for a use that is not protected by patents or legal exclusivities, and must remove all references. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product based on documents submitted by applicants. Drug. How Long Does It Take For A Drug To Be Generic.
From animalia-life.club
How Long Does It Take For A Dogs Body To In The Ground How Long Does It Take For A Drug To Be Generic The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. Drug companies must submit an abbreviated new drug application (called anda) for approval to. Generic versions can be equivalents or authorized generics, which are exact. How are generic drugs approved? A generic drug can be approved for. How Long Does It Take For A Drug To Be Generic.
From www.proactivehealthcare.co.uk
How Long Does It Take to Recover from Vitamin D Deficiency How Long Does It Take For A Drug To Be Generic The fda approves generic drugs through a specific process. Drug companies must submit an abbreviated new drug application (called anda) for approval to. The generic drug approval process in japan in japan, the pmda reviews generic drug applications, and this includes the assessment of bioequivalence studies. This bioequivalence assessment helps to confirm the quality, efficacy, and safety of the product. How Long Does It Take For A Drug To Be Generic.