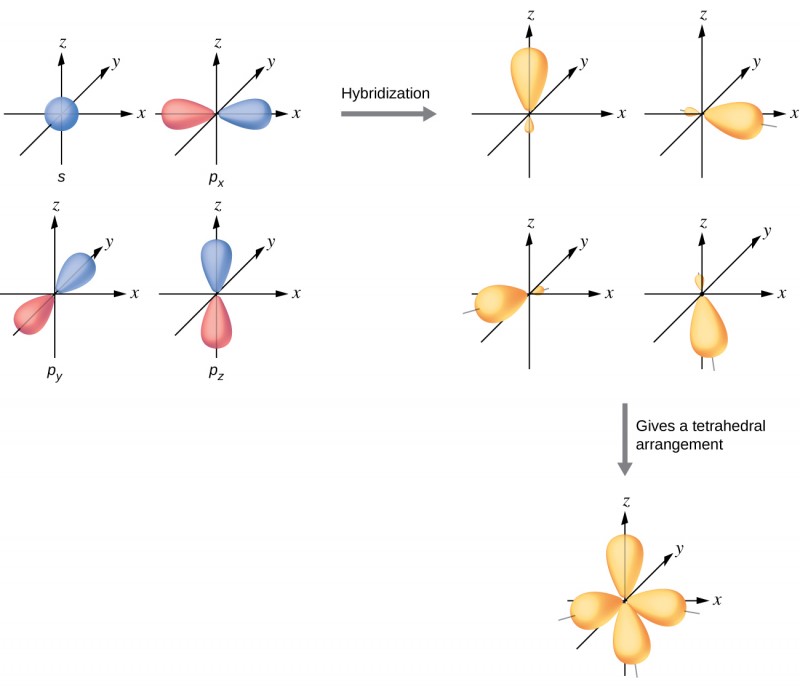
Structure Of Sp3 Hybridization . Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. Find out the types of. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The shape of an sp3 hybridized. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry.
from nigerianscholars.com
In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The shape of an sp3 hybridized. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Find out the types of. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have.
sp3 Hybridization Advanced Theories of Covalent Bonding
Structure Of Sp3 Hybridization In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. The shape of an sp3 hybridized. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. Find out the types of.
From classnotes.org.in
Hybridisation Chemical Bonding and Molecular Structure, Chemistry Structure Of Sp3 Hybridization The shape of an sp3 hybridized. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them. Structure Of Sp3 Hybridization.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Structure Of Sp3 Hybridization The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. Find out the types of. In the new electron configuration, each of. Structure Of Sp3 Hybridization.
From www.nanowerk.com
Understanding SP Hybridization in Nanotechnology Structure Of Sp3 Hybridization The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Find out the types of. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized. Structure Of Sp3 Hybridization.
From www.youtube.com
Hybridization sp3d sp3d2 sp3d3 Formation of PF5, SF6 and Structure Of Sp3 Hybridization Find out the types of. The shape of an sp3 hybridized. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. In the new electron configuration, each of the four valence. Structure Of Sp3 Hybridization.
From avantecnica.qualitypoolsboulder.com
Hybridization Definition, Types, Rules, Examples Structure Of Sp3 Hybridization In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The shape of an sp3 hybridized. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. All the carbon atoms in an alkane are sp3. Structure Of Sp3 Hybridization.
From collegedunia.com
Hybridization sp, sp2, sp3 & sp3d Atomic Orbitals, Properties & Examples Structure Of Sp3 Hybridization In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. The carbons. Structure Of Sp3 Hybridization.
From chemistryskills.com
Orbital Hybridization Chemistry Skills Structure Of Sp3 Hybridization In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. Find out the types of. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. The shape of an sp3 hybridized. All the carbon atoms in an alkane are sp3. Structure Of Sp3 Hybridization.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to Structure Of Sp3 Hybridization All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. The shape of an sp3 hybridized. Find out the types of. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to. Structure Of Sp3 Hybridization.
From chemistryteory.blogspot.com
chemistry Hybridization sp3 hybridization in Methane Structure Of Sp3 Hybridization An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Find out the types of. The shape of an sp3 hybridized. In the new electron configuration, each of the four. Structure Of Sp3 Hybridization.
From eduinput.com
sp3hybridization, definition, explanation, examples and significance Structure Of Sp3 Hybridization The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. Find out the types of. The shape of an sp3 hybridized. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. Learn about the concept of hybridization, the mixing of atomic orbitals to form new. Structure Of Sp3 Hybridization.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Structure Of Sp3 Hybridization The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. Find out. Structure Of Sp3 Hybridization.
From glossary.periodni.com
Sp3 hybrid orbital Chemistry Dictionary & Glossary Structure Of Sp3 Hybridization Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Find out the types of. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form. Structure Of Sp3 Hybridization.
From madoverchemistry.com
62.CHEMICAL BONDING (9) Covalent Bonding(8) sp3 Hybridization Structure Of Sp3 Hybridization Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Find out the types of. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. An atom surrounded by. Structure Of Sp3 Hybridization.
From www.vrogue.co
Chemistry Sp3 Hybrid Orbitals And The Structure Of Me vrogue.co Structure Of Sp3 Hybridization The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Find out the types of. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The carbons in alkenes and other atoms with a double. Structure Of Sp3 Hybridization.
From ar.inspiredpencil.com
Hybridization Orbitals Chart Structure Of Sp3 Hybridization Find out the types of. The shape of an sp3 hybridized. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. The carbons in alkenes and other atoms with a double bond are often sp2. Structure Of Sp3 Hybridization.
From www.youtube.com
sp3, sp2, and sp Hybridization YouTube Structure Of Sp3 Hybridization Find out the types of. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid. Structure Of Sp3 Hybridization.
From www.adda247.com
sp, sp2, sp3 Hybridization Examples, sp3d2 Shape & Structure Structure Of Sp3 Hybridization An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. Find out the types of. The sp3 hybrids have two lobes and. Structure Of Sp3 Hybridization.
From testbook.com
Hybridization of CH4 (Methane) Detailed Explanation and FAQs Structure Of Sp3 Hybridization The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. Find out the types of. The shape. Structure Of Sp3 Hybridization.
From ar.inspiredpencil.com
Hybridization Orbitals Sp Sp2 Sp3 Structure Of Sp3 Hybridization In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Find out the types of. All the carbon atoms in an alkane are sp3 hybridized. Structure Of Sp3 Hybridization.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Structure Of Sp3 Hybridization The shape of an sp3 hybridized. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. Find out the types of. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Learn about the concept of hybridization, the mixing of. Structure Of Sp3 Hybridization.
From classnotes.org.in
Hybridisation Chemical Bonding and Molecular Structure, Chemistry Structure Of Sp3 Hybridization The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. The shape of an sp3. Structure Of Sp3 Hybridization.
From eduinput.com
sp3hybridization, definition, explanation, examples and significance Structure Of Sp3 Hybridization Find out the types of. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. The shape of an sp3 hybridized. In the new electron configuration, each of the four valence electrons on the carbon. Structure Of Sp3 Hybridization.
From www.animalia-life.club
Sp2 Hybridization Shape Structure Of Sp3 Hybridization The shape of an sp3 hybridized. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. In the new electron configuration, each of the. Structure Of Sp3 Hybridization.
From sharedocnow.blogspot.com
Sp Sp2 Sp3 Hybridization sharedoc Structure Of Sp3 Hybridization The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The shape of an sp3 hybridized. An atom surrounded by a tetrahedral arrangement of bonding. Structure Of Sp3 Hybridization.
From www.dreamstime.com
Sp3 Hybridization Illustrated Molecular Structure on White Background Structure Of Sp3 Hybridization The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. All the. Structure Of Sp3 Hybridization.
From www.globalsino.com
sp3 Hybridization Structure Of Sp3 Hybridization In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid. Structure Of Sp3 Hybridization.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Structure Of Sp3 Hybridization Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. Find out the types of. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. The shape of an sp3 hybridized. The sp3. Structure Of Sp3 Hybridization.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Structure Of Sp3 Hybridization The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Find out the types of.. Structure Of Sp3 Hybridization.
From www.collegesearch.in
Sp3 Hybridization Definitions, Examples, Properties, Electronic Structure Of Sp3 Hybridization Find out the types of. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. The shape of an sp3 hybridized. In the new electron configuration, each of the four valence electrons. Structure Of Sp3 Hybridization.
From www.pinterest.jp
Image result for hybridization sp sp2 sp3 and shape Sp sp2 sp3, Química Structure Of Sp3 Hybridization All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. The shape of an sp3 hybridized. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. The carbons in. Structure Of Sp3 Hybridization.
From www.slideserve.com
PPT Chapter 9 PowerPoint Presentation, free download ID4493524 Structure Of Sp3 Hybridization All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. Find out the types of. The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. In the. Structure Of Sp3 Hybridization.
From nigerianscholars.com
sp3 Hybridization Advanced Theories of Covalent Bonding Structure Of Sp3 Hybridization In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. The sp3 hybrids have two lobes and. Structure Of Sp3 Hybridization.
From www.chemistrylibrary.org
Sp3 hybridization Structure Of Sp3 Hybridization The sp3 hybrids have two lobes and are unsymmetrical about the nucleus, giving them a directionality and allowing them to form strong. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. Find out the. Structure Of Sp3 Hybridization.
From trynstopus.blogspot.com
Try and stop us May 2006 Structure Of Sp3 Hybridization Learn about the concept of hybridization, the mixing of atomic orbitals to form new hybrid orbitals. Find out the types of. The carbons in alkenes and other atoms with a double bond are often sp2 hybridized and have. All the carbon atoms in an alkane are sp3 hybridized with tetrahedral geometry. An atom surrounded by a tetrahedral arrangement of bonding. Structure Of Sp3 Hybridization.
From ar.inspiredpencil.com
Ch4 Hybridization Structure Of Sp3 Hybridization Find out the types of. In the new electron configuration, each of the four valence electrons on the carbon occupies a single sp3 orbital creating four unpaired electrons. The shape of an sp3 hybridized. An atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs has a set of four. Learn about the concept of hybridization, the mixing. Structure Of Sp3 Hybridization.