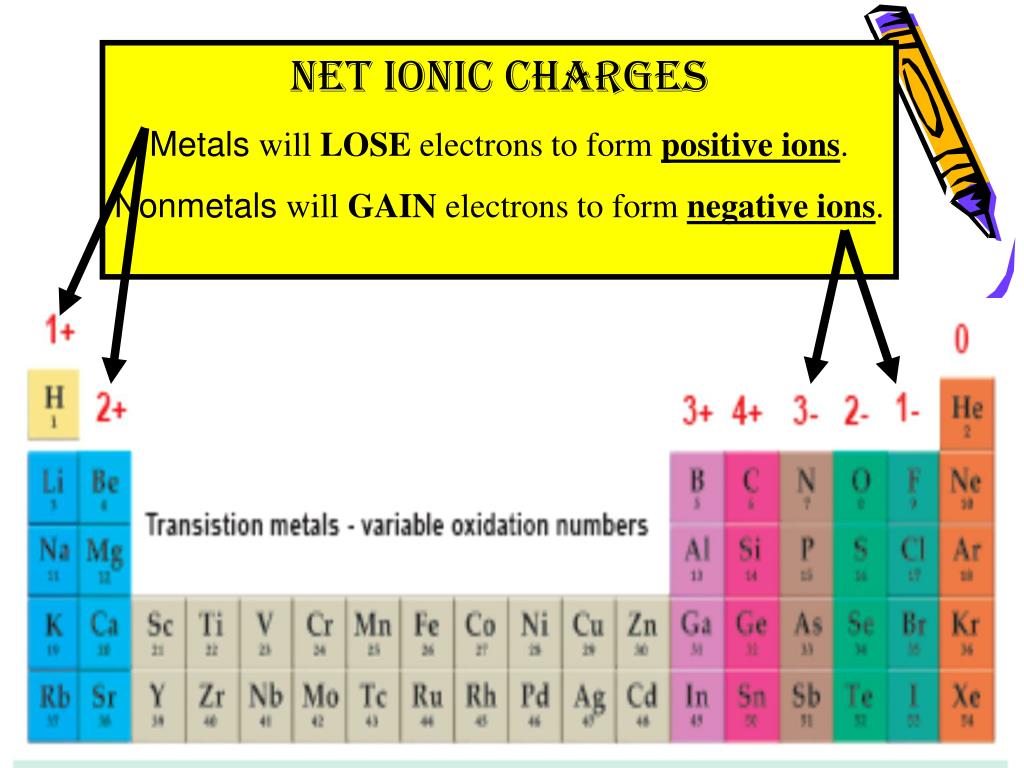
Tin Gain Electrons . Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. 93 rows this table shows the most common charges for atoms of the chemical elements. Sncl 2 forms both sncl 4 and tin metal. Define the two types of ions. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Tin has 4 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Does tin gain or lose electrons in this reaction?
from www.slideserve.com
Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Sncl 2 forms both sncl 4 and tin metal. Define the two types of ions. Tin has 4 valence electrons. Does tin gain or lose electrons in this reaction? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. 93 rows this table shows the most common charges for atoms of the chemical elements.
PPT Structure & Properties of Matter PowerPoint Presentation ID5671688
Tin Gain Electrons Define the two types of ions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Sncl 2 forms both sncl 4 and tin metal. 93 rows this table shows the most common charges for atoms of the chemical elements. Tin has 4 valence electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Define the two types of ions. Does tin gain or lose electrons in this reaction? Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s.
From www.dreamstime.com
Atom of Tin with Detailed Core and Its 50 Electrons with Atoms Stock Tin Gain Electrons Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. 93 rows this table shows the most common charges for atoms of the chemical elements. Define the two types of ions. Does tin gain or lose electrons in this reaction? Atoms tend to lose, gain, or. Tin Gain Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Tin (Sn, Sn2+, Sn4+) Tin Gain Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Sncl 2 forms both sncl 4 and tin metal. Tin has 4 valence electrons. Does tin gain or lose electrons in this reaction? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or.. Tin Gain Electrons.
From courses.lumenlearning.com
Electrons Biology for Majors I Tin Gain Electrons Does tin gain or lose electrons in this reaction? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Tin has 4 valence electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. Sncl 2 forms both sncl 4 and tin metal. Finally,. Tin Gain Electrons.
From www.slideserve.com
PPT Nomenclature of Compounds PowerPoint Presentation, free Tin Gain Electrons Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Does tin gain or lose electrons in this reaction? Atoms tend to lose, gain, or. Tin Gain Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Gain Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Define the two types of ions. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Does tin. Tin Gain Electrons.
From socratic.org
How can you tell if an element wants to gain or lose electrons? Socratic Tin Gain Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. Define the two types of ions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's. Tin Gain Electrons.
From valenceelectrons.com
How to find the Protons, Neutrons and Electrons for Tin? Tin Gain Electrons Does tin gain or lose electrons in this reaction? Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Tin has 4 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. 93 rows this table shows the most common charges. Tin Gain Electrons.
From www.researchgate.net
Band structures of a) MoS 2 and b) MoS 2 TiN. c) Bader charge transfer Tin Gain Electrons Tin has 4 valence electrons. Define the two types of ions. Sncl 2 forms both sncl 4 and tin metal. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Does tin gain. Tin Gain Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Tin (Sn)? Tin Gain Electrons Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Tin has 4 valence electrons. Does tin gain or lose electrons in this reaction? 93 rows this table shows the. Tin Gain Electrons.
From ar.inspiredpencil.com
Tin Atomic Structure Tin Gain Electrons Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Sncl 2 forms both sncl 4 and tin metal. 93 rows this table shows the most common charges for atoms of the chemical elements. Does tin gain or lose electrons in this reaction? 93 rows you. Tin Gain Electrons.
From giogmbpmr.blob.core.windows.net
Does Tin Gain Electrons Forming Anions at Carlos Mercer blog Tin Gain Electrons Sncl 2 forms both sncl 4 and tin metal. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Tin has 4 valence electrons. Does tin gain or lose electrons in this reaction? Atoms tend to lose, gain, or share some valance electrons, making bonds to. Tin Gain Electrons.
From slideplayer.com
Notes Ionic Bonds 1. Key Concept Ionic bonds form when electrons are Tin Gain Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the. Tin Gain Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Tin Have? Tin Gain Electrons Sncl 2 forms both sncl 4 and tin metal. Define the two types of ions. Does tin gain or lose electrons in this reaction? Tin has 4 valence electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler. Tin Gain Electrons.
From www.youtube.com
How to Find the Valence Electrons for Tin (Sn) YouTube Tin Gain Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s.. Tin Gain Electrons.
From www.slideserve.com
PPT IV. Chemical Bonding PowerPoint Presentation, free download ID Tin Gain Electrons Does tin gain or lose electrons in this reaction? 93 rows this table shows the most common charges for atoms of the chemical elements. Define the two types of ions. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. 93 rows you may assume the valences of the chemical elements—the number of electrons. Tin Gain Electrons.
From www.numerade.com
SOLVED Examining your labeled periodic table, which of the following Tin Gain Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Tin has 4 valence electrons. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Tin Gain Electrons.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Sulfur vs Vanadium Tin Gain Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. 93 rows you may assume. Tin Gain Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Gain Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Sncl 2 forms both sncl 4 and tin metal. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Does tin gain or lose electrons in this reaction? 93 rows this table shows. Tin Gain Electrons.
From juliannanewsbradford.blogspot.com
How Many Electrons Are in a Tin Atom Tin Gain Electrons Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Tin has 4 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Does tin gain or lose electrons in this reaction? Define. Tin Gain Electrons.
From juliannanewsbradford.blogspot.com
How Many Electrons Are in a Tin Atom Tin Gain Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Sncl 2 forms both sncl 4 and tin metal. Define the two types of ions. 93 rows this table shows the most common charges for atoms of the chemical elements. Tin has 4 valence electrons. Does tin gain or lose electrons in this reaction?. Tin Gain Electrons.
From www.youtube.com
Electrons in Atoms Lesson7 Noble Gas Configuration YouTube Tin Gain Electrons Does tin gain or lose electrons in this reaction? Sncl 2 forms both sncl 4 and tin metal. Tin has 4 valence electrons. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. 93 rows this table shows the most common charges for atoms of the. Tin Gain Electrons.
From brainly.com
is this atom more likely to gain or lose electrons? explain how you can Tin Gain Electrons Sncl 2 forms both sncl 4 and tin metal. 93 rows this table shows the most common charges for atoms of the chemical elements. Tin has 4 valence electrons. Define the two types of ions. Does tin gain or lose electrons in this reaction? 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Tin Gain Electrons.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Tin Gain Electrons Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Sncl 2 forms both sncl 4 and tin metal. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Atoms tend to lose, gain, or. Tin Gain Electrons.
From www.slideshare.net
10 28 How Many Electrons Do Atoms Gain Lose Tin Gain Electrons 93 rows this table shows the most common charges for atoms of the chemical elements. Define the two types of ions. Sncl 2 forms both sncl 4 and tin metal. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Atoms tend to lose, gain, or. Tin Gain Electrons.
From www.dreamstime.com
Cations and Anions Electrons Gain Lose Stock Vector Illustration of Tin Gain Electrons Define the two types of ions. Tin has 4 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Does tin gain or lose electrons in this reaction? Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic. Tin Gain Electrons.
From www.alamy.com
3d render of atom structure of tin isolated over white background Tin Gain Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Sncl 2 forms both sncl 4 and tin metal. 93 rows this table shows the most common charges for atoms of the chemical elements. Tin has 4 valence electrons. Define the two types of ions. Atoms tend to lose,. Tin Gain Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Gain Electrons Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Tin has 4 valence electrons. Define the two types of ions. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Sncl 2 forms both. Tin Gain Electrons.
From www.vrogue.co
Calculate The Number Of Electrons Lost Or Gained Duri vrogue.co Tin Gain Electrons Does tin gain or lose electrons in this reaction? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Define the two types of ions. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s.. Tin Gain Electrons.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Tin Gain Electrons Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. 93 rows this table shows the most common charges for atoms of the chemical elements. Does tin gain or lose electrons in this reaction? 93 rows you may assume the valences of the chemical elements—the number. Tin Gain Electrons.
From www.numerade.com
SOLVED Which image below best illustrates what happens at the Tin Gain Electrons Tin has 4 valence electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. Does tin gain or lose electrons in this reaction? Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Define the two types of ions. Sncl 2 forms both sncl 4 and tin metal.. Tin Gain Electrons.
From valenceelectrons.com
Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+) Tin Gain Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Sncl 2 forms both sncl 4 and tin metal. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. Tin has 4 valence electrons. Does tin gain or lose electrons. Tin Gain Electrons.
From www.nagwa.com
Question Video Determining the Number of Electrons an Atom of Fluorine Tin Gain Electrons Tin has 4 valence electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Finally, according to the orbital potential energies listed on page 16 of appendix b of miessler and tarr's inorganic chemistry (5th ed.), the 5s. 93 rows this table shows the most common charges for atoms of the chemical elements.. Tin Gain Electrons.
From www.slideserve.com
PPT Structure & Properties of Matter PowerPoint Presentation ID5671688 Tin Gain Electrons Does tin gain or lose electrons in this reaction? Sncl 2 forms both sncl 4 and tin metal. Define the two types of ions. Tin has 4 valence electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Tin Gain Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Gain Electrons Does tin gain or lose electrons in this reaction? 93 rows this table shows the most common charges for atoms of the chemical elements. Tin has 4 valence electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Define the two types of ions. Finally, according to the orbital potential energies listed on. Tin Gain Electrons.
From www.numerade.com
SOLVED Consideran electrochemical cell irvolving Silver and Tin IAg Tin Gain Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. 93 rows this table shows the most common charges for atoms of the chemical elements. Define the two types of ions. Does tin gain or lose electrons in this reaction? 93 rows you may assume the valences of the chemical elements—the number of electrons. Tin Gain Electrons.