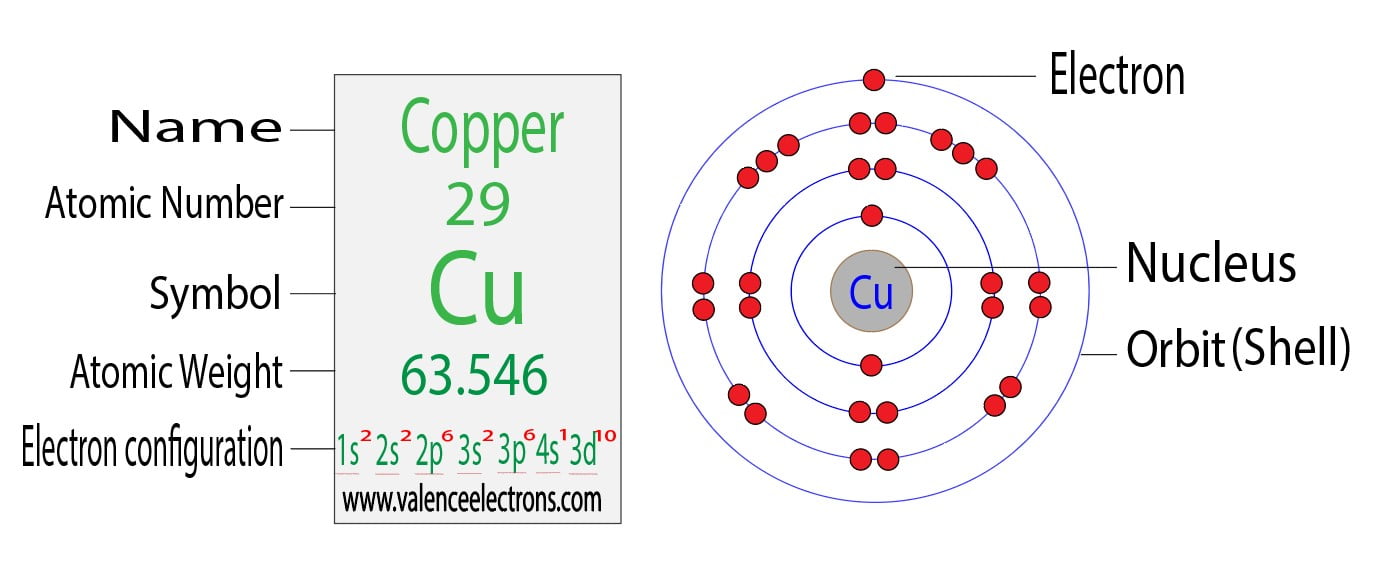
Copper 2 Valence Electrons . Valence electrons only include the electrons in the highest energy (n). Since 1s can only hold two electrons the next 2. Yes, copper only has 1 valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. For this, copper ion(cu + ). Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). How to find the valence electrons of a chosen element? In writing the electron configuration for copper the first two electrons will go in the 1s orbital. You can check it immediately in our valence electron calculator on the left or.
from valenceelectrons.com
How to find the valence electrons of a chosen element? In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. You can check it immediately in our valence electron calculator on the left or. Valence electrons only include the electrons in the highest energy (n). The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). But for most of the transition. For this, copper ion(cu + ). Since 1s can only hold two electrons the next 2.
Electron Configuration for Copper (Cu, Cu+, Cu2+)
Copper 2 Valence Electrons In writing the electron configuration for copper the first two electrons will go in the 1s orbital. For this, copper ion(cu + ). Since 1s can only hold two electrons the next 2. How to find the valence electrons of a chosen element? But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Yes, copper only has 1 valence electron. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Valence electrons only include the electrons in the highest energy (n). You can check it immediately in our valence electron calculator on the left or. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10).
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper 2 Valence Electrons Valence electrons only include the electrons in the highest energy (n). Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and. Copper 2 Valence Electrons.
From reviewhomedecor.co
Copper Periodic Table Electrons Review Home Decor Copper 2 Valence Electrons How to find the valence electrons of a chosen element? The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Since 1s. Copper 2 Valence Electrons.
From strongnewsupdates.s3.amazonaws.com
Copper Valence Electrons Copper 2 Valence Electrons Yes, copper only has 1 valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons only include the electrons in the highest energy (n). How to find the valence electrons of a chosen element? Valence electrons are the electrons that reside in. Copper 2 Valence Electrons.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper 2 Valence Electrons For this, copper ion(cu + ). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons only include the electrons in the highest energy (n). Since 1s can only hold two electrons the next 2. Yes, copper only has 1 valence electron. You can. Copper 2 Valence Electrons.
From periodictable.me
Copper Valence Electrons Archives Dynamic Periodic Table of Elements and Chemistry Copper 2 Valence Electrons Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Since 1s can only hold two electrons the next 2. For this, copper ion(cu + ). The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last. Copper 2 Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper 2 Valence Electrons In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). Valence electrons only include the electrons in the highest energy (n). But for most of the transition.. Copper 2 Valence Electrons.
From wiringguidefrosts.z19.web.core.windows.net
Copper Electron Configuration Diagram Copper 2 Valence Electrons Valence electrons only include the electrons in the highest energy (n). For this, copper ion(cu + ). Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. But for most of the transition. In writing the electron configuration for copper the first two. Copper 2 Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Lithium (Li)? Copper 2 Valence Electrons In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2. The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). The electron distribution in copper spans across. Copper 2 Valence Electrons.
From ar.inspiredpencil.com
How Many Valence Electrons Does Copper Have Copper 2 Valence Electrons How to find the valence electrons of a chosen element? You can check it immediately in our valence electron calculator on the left or. But for most of the transition. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron configuration of copper ion. Copper 2 Valence Electrons.
From printableshub.com
Free Printable Periodic Table (With names, charges & Valence Electrons) [PDF] Printables Hub Copper 2 Valence Electrons Valence electrons only include the electrons in the highest energy (n). Yes, copper only has 1 valence electron. But for most of the transition. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. You can check it immediately in our valence electron calculator on the. Copper 2 Valence Electrons.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Copper (Cu) YouTube Copper 2 Valence Electrons The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). But for most of the transition. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Valence electrons are the electrons that reside in the outermost energy. Copper 2 Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Copper 2 Valence Electrons But for most of the transition. You can check it immediately in our valence electron calculator on the left or. Valence electrons only include the electrons in the highest energy (n). Yes, copper only has 1 valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Copper 2 Valence Electrons.
From mavink.com
Periodic Table Valence Electrons Copper 2 Valence Electrons Yes, copper only has 1 valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Valence electrons only include the electrons in the highest energy (n). The electron configuration of copper ion shows that copper ion(cu +) has three shells and. Copper 2 Valence Electrons.
From ar.inspiredpencil.com
Copper Orbital Diagram Copper 2 Valence Electrons You can check it immediately in our valence electron calculator on the left or. Since 1s can only hold two electrons the next 2. How to find the valence electrons of a chosen element? In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Valence electrons are the electrons that reside in the. Copper 2 Valence Electrons.
From electraschematics.com
Understanding the Electron Configuration Diagram for Copper Copper 2 Valence Electrons The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). You can check it immediately in our valence electron calculator on the left or. Since 1s can only hold two electrons the next 2. Yes, copper only has 1 valence electron. How to find. Copper 2 Valence Electrons.
From guide-scientific.com
How many valence electrons does Copper (Cu) have? Copper valence. Copper 2 Valence Electrons For this, copper ion(cu + ). Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Yes, copper only has 1 valence electron. How to find the valence electrons of a chosen element? The electron configuration of copper ion shows that copper ion(cu. Copper 2 Valence Electrons.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Copper 2 Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). Since 1s can only hold two electrons the next 2.. Copper 2 Valence Electrons.
From www.allaboutcircuits.com
Valence and Crystal Structure Solidstate Device Theory Electronics Textbook Copper 2 Valence Electrons But for most of the transition. The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). Yes, copper only has 1 valence electron. Since 1s can only hold two electrons the next 2. The electron distribution in copper spans across the k, l, m,. Copper 2 Valence Electrons.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration, chemical data, and Copper 2 Valence Electrons The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Yes, copper only has 1 valence electron. For this, copper ion(cu + ). But for most of the transition. How to find the valence electrons of a chosen element? The electron configuration of copper ion shows. Copper 2 Valence Electrons.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper 2 Valence Electrons Valence electrons only include the electrons in the highest energy (n). But for most of the transition. How to find the valence electrons of a chosen element? For this, copper ion(cu + ). The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Yes, copper only. Copper 2 Valence Electrons.
From www.alamy.com
Copper (Cu). Diagram of the valence orbitals of an atom of copper64 (atomic number 29), an Copper 2 Valence Electrons Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In writing the electron configuration for copper the first. Copper 2 Valence Electrons.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper 2 Valence Electrons Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. For this, copper ion(cu + ). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The electron configuration. Copper 2 Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Copper 2 Valence Electrons In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Valence electrons only include the electrons in the highest energy (n). Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. For this, copper ion(cu +. Copper 2 Valence Electrons.
From www.researchgate.net
What is Electron Configuration for Cu+2? ResearchGate Copper 2 Valence Electrons Since 1s can only hold two electrons the next 2. The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen electrons(3s 2 3p 6 3d 10). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Copper 2 Valence Electrons.
From ar.inspiredpencil.com
Electron Configuration Of Copper Copper 2 Valence Electrons Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. You can check it immediately in our valence electron calculator on the left or. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will. Copper 2 Valence Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Copper Have? Copper 2 Valence Electrons You can check it immediately in our valence electron calculator on the left or. For this, copper ion(cu + ). How to find the valence electrons of a chosen element? Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Since 1s can. Copper 2 Valence Electrons.
From www.youtube.com
Electron Configuration for Cu, Cu+, and Cu2+ (Copper and Copper Ions) YouTube Copper 2 Valence Electrons Yes, copper only has 1 valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. But for most of the transition. The electron configuration of copper ion shows that copper ion(cu +) has three shells and the last shell has eighteen. Copper 2 Valence Electrons.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Copper 2 Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. How to find the valence electrons of a chosen element? In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Valence electrons only include the electrons in the highest. Copper 2 Valence Electrons.
From www.youtube.com
How to find Protons & Electrons for Cu+ and Cu2+ (Copper II and III ions) YouTube Copper 2 Valence Electrons Since 1s can only hold two electrons the next 2. Yes, copper only has 1 valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. But for most of the transition. For this, copper ion(cu + ). You can check it immediately in our. Copper 2 Valence Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper 2 Valence Electrons How to find the valence electrons of a chosen element? Since 1s can only hold two electrons the next 2. For this, copper ion(cu + ). Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. In writing the electron configuration for copper. Copper 2 Valence Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper II Easy & Quick YouTube Copper 2 Valence Electrons You can check it immediately in our valence electron calculator on the left or. Since 1s can only hold two electrons the next 2. For this, copper ion(cu + ). How to find the valence electrons of a chosen element? In writing the electron configuration for copper the first two electrons will go in the 1s orbital. The electron configuration. Copper 2 Valence Electrons.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Copper 2 Valence Electrons Since 1s can only hold two electrons the next 2. You can check it immediately in our valence electron calculator on the left or. Yes, copper only has 1 valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons are the electrons. Copper 2 Valence Electrons.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper 2 Valence Electrons The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. How to find the valence electrons of a chosen element? Since 1s can only hold two electrons the next 2. Yes, copper only has 1 valence electron. The electron configuration of copper ion shows that copper. Copper 2 Valence Electrons.
From readingandwritingprojectcom.web.fc2.com
valence electrons of copper Copper 2 Valence Electrons Yes, copper only has 1 valence electron. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Valence electrons only include the electrons in the highest. Copper 2 Valence Electrons.
From www.alamy.com
3d render of atom structure of copper isolated over white background Stock Photo 137220153 Alamy Copper 2 Valence Electrons You can check it immediately in our valence electron calculator on the left or. How to find the valence electrons of a chosen element? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons only include the electrons in the highest energy (n). But. Copper 2 Valence Electrons.