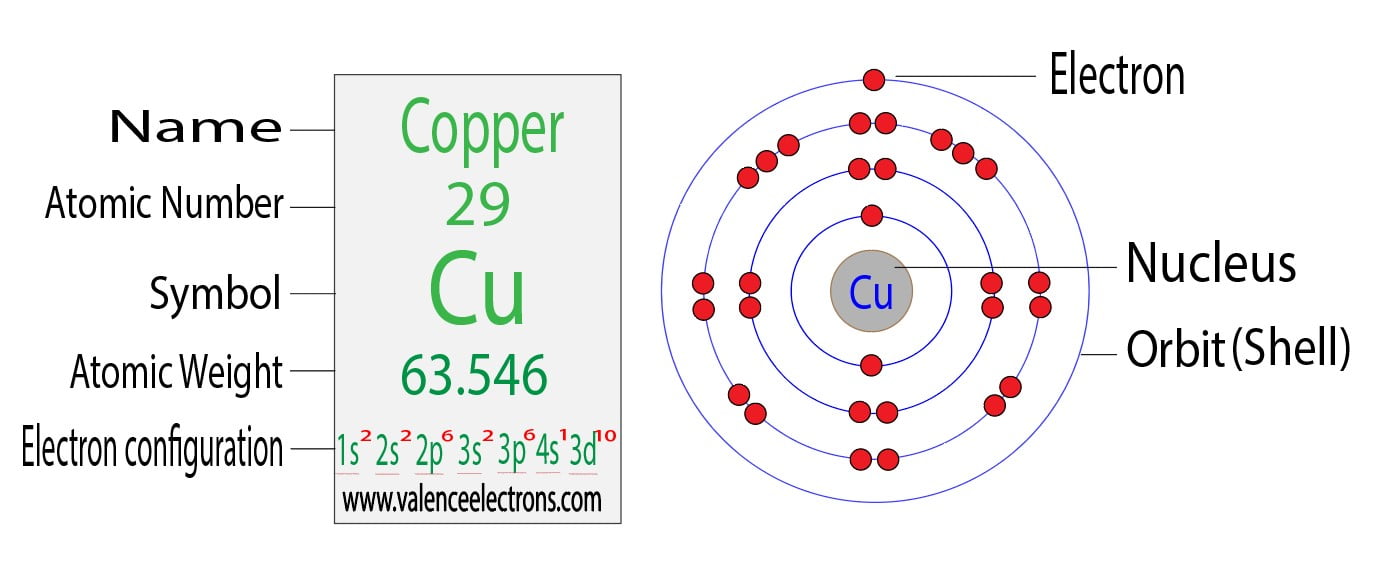
Does Copper Take Electrons . Possible oxidation states are +1,2. It will give up that one electron under a weakly charged. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. Since 1s can only hold two electrons the next 2. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. A sheet of aluminum foil and a copper wire are both places where you can. But at the same time, copper ions. It has the following electron count in each layer: Consider the atom of copper. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Electron configuration of copper is [ar] 3d10 4s1. According to my book, metals want to lose electrons to achieve noble gas configuration. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively.
from ar.inspiredpencil.com
But at the same time, copper ions. A sheet of aluminum foil and a copper wire are both places where you can. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. It has the following electron count in each layer: The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Possible oxidation states are +1,2. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. Consider the atom of copper. According to my book, metals want to lose electrons to achieve noble gas configuration.
Orbital Diagram For Copper
Does Copper Take Electrons It has the following electron count in each layer: According to my book, metals want to lose electrons to achieve noble gas configuration. Since 1s can only hold two electrons the next 2. It will give up that one electron under a weakly charged. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. Possible oxidation states are +1,2. Electron configuration of copper is [ar] 3d10 4s1. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. But at the same time, copper ions. A sheet of aluminum foil and a copper wire are both places where you can. Consider the atom of copper. It has the following electron count in each layer:
From mccnsulting.web.fc2.com
How many electrons does copper have? Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. But at the same time, copper ions. It has the following electron count in each layer: Consider the atom of copper. According to my book, metals want to lose electrons to achieve noble gas configuration. It will give up that one electron under a weakly. Does Copper Take Electrons.
From www.slideserve.com
PPT Basic Electrical Theory PowerPoint Presentation, free download ID6633003 Does Copper Take Electrons The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. But at the same time, copper ions. Since 1s can only hold two electrons the next 2. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons. Does Copper Take Electrons.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Does Copper Take Electrons In writing the electron configuration for copper the first two electrons will go in the 1s orbital. According to my book, metals want to lose electrons to achieve noble gas configuration. It will give up that one electron under a weakly charged. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free. Does Copper Take Electrons.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration, chemical data, and Does Copper Take Electrons It will give up that one electron under a weakly charged. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper forms a rich variety of compounds, usually. Does Copper Take Electrons.
From mybios.me
Copper Periodic Table Protons Neutrons And Electrons Bios Pics Does Copper Take Electrons Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. Electron configuration of copper is [ar] 3d10 4s1. It will give up that one electron under a weakly charged. According to my book, metals want to lose electrons to achieve noble gas configuration. Consider the atom. Does Copper Take Electrons.
From www.numerade.com
SOLVED The density of mobile electrons in copper metal Is 8.4 1028 m 3 . Suppose that / = 2.8 Does Copper Take Electrons It has the following electron count in each layer: Since 1s can only hold two electrons the next 2. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. Consider the atom of copper. But at the same time, copper ions. In writing the electron configuration. Does Copper Take Electrons.
From elchoroukhost.net
Copper Periodic Table Valence Electrons Elcho Table Does Copper Take Electrons Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. Since 1s can only hold two electrons the next 2. It will give up that one electron under a weakly charged. It has the following electron count in each layer: A sheet of aluminum foil and. Does Copper Take Electrons.
From material-properties.org
Copper Protons Neutrons Electrons Electron Configuration Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. Electron configuration of copper is [ar] 3d10 4s1. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Since 1s can only hold two electrons the next 2. In writing the. Does Copper Take Electrons.
From stock.adobe.com
Periodic Table of the Elements, Shell Structure of Copper Cu Electrons per energy level. Stock Does Copper Take Electrons According to my book, metals want to lose electrons to achieve noble gas configuration. Since 1s can only hold two electrons the next 2. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. But at the same time, copper ions. A sheet of aluminum foil and a copper wire are both places where you can.. Does Copper Take Electrons.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Does Copper Take Electrons According to my book, metals want to lose electrons to achieve noble gas configuration. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. But at the same time, copper ions. Electron configuration of copper is [ar] 3d10 4s1. The electron distribution in copper spans across. Does Copper Take Electrons.
From www.chegg.com
Solved The freeelectron density in a copper wire is Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. It has the following electron count in each layer: It will give up that one electron under a weakly charged. Possible oxidation states are +1,2. Since 1s can only hold two electrons the next 2. The electron distribution in copper spans across the k, l,. Does Copper Take Electrons.
From www.nuclear-power.com
Copper Electron Affinity Electronegativity Ionization Energy of Copper Does Copper Take Electrons Consider the atom of copper. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Electron configuration of copper is [ar] 3d10. Does Copper Take Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Does Copper Take Electrons Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. It will give up that one electron under a weakly charged. Consider the atom of copper. According to my book, metals want to lose electrons to achieve noble gas configuration. It has the following electron count in each layer: Electron configuration of copper is [ar] 3d10. Does Copper Take Electrons.
From www.alamy.com
Copper atom hires stock photography and images Alamy Does Copper Take Electrons Consider the atom of copper. A sheet of aluminum foil and a copper wire are both places where you can. It will give up that one electron under a weakly charged. According to my book, metals want to lose electrons to achieve noble gas configuration. It has the following electron count in each layer: But at the same time, copper. Does Copper Take Electrons.
From aliceandallthatjazz.blogspot.com
Electron Configuration Of Copper 1+ worksheet Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. Possible oxidation states are +1,2. In writing the electron configuration for copper the first two electrons will go in the 1s. Does Copper Take Electrons.
From www.earthdate.org
Copper’s Superpower EarthDate Does Copper Take Electrons But at the same time, copper ions. Consider the atom of copper. It will give up that one electron under a weakly charged. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Since 1s can only hold two electrons the next 2. Possible oxidation states are +1,2.. Does Copper Take Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Does Copper Take Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Possible oxidation states are +1,2. A sheet of aluminum foil and a copper wire are both places where you can. Electron configuration of copper is [ar] 3d10 4s1. According to my book, metals want to lose electrons to. Does Copper Take Electrons.
From slideplayer.com
Structure of the Atom Chapter 19 Section ppt download Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. But at the same time, copper ions. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. It will give up that one electron under a weakly charged. Electron configuration of. Does Copper Take Electrons.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Does Copper Take Electrons The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. It will give up that one electron under a weakly charged. Electron configuration of copper is [ar] 3d10 4s1. Copper forms a. Does Copper Take Electrons.
From www.youtube.com
ELECTRONIC CONFIGURATION OF COPPER ATOM and STABILITY YouTube Does Copper Take Electrons In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. It has the following electron. Does Copper Take Electrons.
From www.mooramo.com
Ions of Transition Elements Mooramo Does Copper Take Electrons Since 1s can only hold two electrons the next 2. But at the same time, copper ions. A sheet of aluminum foil and a copper wire are both places where you can. Possible oxidation states are +1,2. According to my book, metals want to lose electrons to achieve noble gas configuration. It will give up that one electron under a. Does Copper Take Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Copper (Cu) YouTube Does Copper Take Electrons Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and. Does Copper Take Electrons.
From www.britannica.com
Copper Uses, Properties, & Facts Britannica Does Copper Take Electrons Consider the atom of copper. It has the following electron count in each layer: In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Possible oxidation states are +1,2. Copper compounds,. Does Copper Take Electrons.
From www.go4prep.com
Copper Electron configuration, Atomic number, Mass, Uses Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. It has the following electron count in each layer: According to my book, metals want to lose electrons to achieve noble gas configuration. Possible oxidation states are +1,2. It will give up that one electron under a weakly charged. Electron configuration of copper is [ar]. Does Copper Take Electrons.
From www.dreamstime.com
Electron of the Element Copper Stock Vector Illustration of configuration, element 251040446 Does Copper Take Electrons But at the same time, copper ions. It will give up that one electron under a weakly charged. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Electron configuration of copper is [ar] 3d10 4s1. According to my book, metals want to lose electrons to achieve noble. Does Copper Take Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper II Easy & Quick YouTube Does Copper Take Electrons It has the following electron count in each layer: Electron configuration of copper is [ar] 3d10 4s1. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s. Does Copper Take Electrons.
From slideplayer.com
Chapter 10 Electrochemistry. ppt download Does Copper Take Electrons It has the following electron count in each layer: Since the wire is made of a conductive material, such as copper, its constituent atoms have many free electrons which can easily move through. Since 1s can only hold two electrons the next 2. Consider the atom of copper. Electron configuration of copper is [ar] 3d10 4s1. But at the same. Does Copper Take Electrons.
From www.alamy.com
Symbol and electron diagram of Copper illustration Stock Vector Image & Art Alamy Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. It has the following electron count in each layer: But at the same time, copper ions. According to my book, metals want to. Does Copper Take Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. It will give up that one electron under a weakly charged. It has the following electron count in each layer: Electron configuration of copper is [ar] 3d10 4s1. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. Consider the atom. Does Copper Take Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Does Copper Take Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. In writing the electron configuration for copper the first two electrons will go in the 1s orbital. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. Electron configuration of copper is [ar] 3d10. Does Copper Take Electrons.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Does Copper Take Electrons According to my book, metals want to lose electrons to achieve noble gas configuration. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. It will give up that one electron under. Does Copper Take Electrons.
From ar.inspiredpencil.com
Orbital Diagram For Copper Does Copper Take Electrons A sheet of aluminum foil and a copper wire are both places where you can. Possible oxidation states are +1,2. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Since 1s can only hold two electrons the next 2. According to my book, metals want. Does Copper Take Electrons.
From donghokiddy.com
Does Copper Really Have 3 Valence Electrons? Exploring Its Atomic Properties Does Copper Take Electrons Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. According to my book, metals want to lose electrons to achieve noble gas configuration. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. Since the wire is made of a conductive material, such. Does Copper Take Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Copper Have? Does Copper Take Electrons Since 1s can only hold two electrons the next 2. It will give up that one electron under a weakly charged. Consider the atom of copper. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In writing the electron configuration for copper the first two. Does Copper Take Electrons.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Does Copper Take Electrons According to my book, metals want to lose electrons to achieve noble gas configuration. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological. Consider the atom of copper. Electron configuration of copper is [ar] 3d10 4s1. It has the following electron count in each layer: But at the same time, copper ions. The electron distribution. Does Copper Take Electrons.