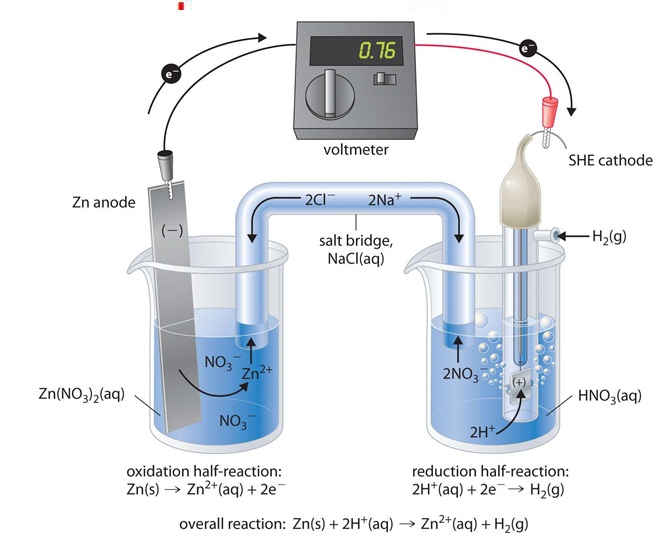
Platinum Electrode Definition In Chemistry . This page explains the background to standard. platinum is the most widely used electrode material. an introduction to redox equilibria and electrode potentials. Platinum has higher charge injection capability than gold and is. The surface of platinum can. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. Among these, platinum is likely the favorite, demonstrating. You can order pt as a wire of various. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. — platinum has catalytic qualities which promote the proton reduction reaction. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an.
from dxontbmym.blob.core.windows.net
the most commonly used working electrode materials are platinum, gold, carbon, and mercury. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. Platinum has higher charge injection capability than gold and is. platinum is the most widely used electrode material. The surface of platinum can. — platinum has catalytic qualities which promote the proton reduction reaction. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Among these, platinum is likely the favorite, demonstrating. You can order pt as a wire of various.
Electrode Meaning Chemistry at Christy Rogers blog
Platinum Electrode Definition In Chemistry — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. You can order pt as a wire of various. Platinum has higher charge injection capability than gold and is. — platinum has catalytic qualities which promote the proton reduction reaction. This page explains the background to standard. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. The surface of platinum can. Among these, platinum is likely the favorite, demonstrating. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. an introduction to redox equilibria and electrode potentials. as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. platinum is the most widely used electrode material.
From periodictable.com
Platinum electrode, a sample of the element Platinum in the Periodic Table Platinum Electrode Definition In Chemistry Platinum has higher charge injection capability than gold and is. This page explains the background to standard. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. platinum is the most widely used electrode material. You can order pt as a wire of various. The surface of platinum can.. Platinum Electrode Definition In Chemistry.
From www.gexin.in
PLATINUM ELECTRODES Asha Scientific Works Platinum Electrode Definition In Chemistry an introduction to redox equilibria and electrode potentials. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. You can order pt as a wire of various. The surface of platinum can. she is composed of a 1.0 m h + (aq) solution containing a square piece of. Platinum Electrode Definition In Chemistry.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Platinum Electrode Definition In Chemistry the most commonly used working electrode materials are platinum, gold, carbon, and mercury. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. This page explains the background to standard. — platinum has catalytic qualities which promote the proton reduction reaction. revision notes on 5.4.2 standard electrode. Platinum Electrode Definition In Chemistry.
From www.deltaed.co.nz
Electrodes Platinum Pair Hoffman Voltame Delta Educational Platinum Electrode Definition In Chemistry Among these, platinum is likely the favorite, demonstrating. Platinum has higher charge injection capability than gold and is. The surface of platinum can. — platinum has catalytic qualities which promote the proton reduction reaction. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where. Platinum Electrode Definition In Chemistry.
From www.knowledgeboat.com
Chapter 6 Electrolysis Selina Solutions Concise Chemistry Class 10 Platinum Electrode Definition In Chemistry revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. — platinum has catalytic qualities which promote the proton reduction reaction. platinum is the most widely used electrode material. Platinum has higher charge injection capability than gold and is. an introduction to redox. Platinum Electrode Definition In Chemistry.
From mavink.com
Electrode Classification Diagram Platinum Electrode Definition In Chemistry — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. You can order pt as a wire of various. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
What reaction occurs at the platinum counter electrode in nonaqueous 3 Platinum Electrode Definition In Chemistry This page explains the background to standard. — platinum has catalytic qualities which promote the proton reduction reaction. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. Platinum has higher charge injection capability than gold and is. platinum is the most widely used electrode material. The surface of platinum can. revision notes. Platinum Electrode Definition In Chemistry.
From ravindraheraeus.com
Platinum Electrodes Ravindra Heraeus Platinum Electrode Definition In Chemistry — platinum has catalytic qualities which promote the proton reduction reaction. Platinum has higher charge injection capability than gold and is. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. the most commonly used working electrode materials are platinum,. Platinum Electrode Definition In Chemistry.
From www.philipharris.co.uk
Platinum Electrodes B8A37126 Philip Harris Platinum Electrode Definition In Chemistry the most commonly used working electrode materials are platinum, gold, carbon, and mercury. The surface of platinum can. an introduction to redox equilibria and electrode potentials. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. she is composed of a 1.0 m h + (aq) solution. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
IV characteristics of the overhanging platinum electrodes (cross Platinum Electrode Definition In Chemistry as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. Platinum has higher charge injection capability than gold and is. — platinum has catalytic qualities which promote the proton reduction reaction. revision notes on 5.4.2 standard electrode. Platinum Electrode Definition In Chemistry.
From www.youtube.com
Describe Construction and Working Of Rotating platinum micro electrode Platinum Electrode Definition In Chemistry as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. This page explains the background to standard. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. — platinum has catalytic qualities which promote. Platinum Electrode Definition In Chemistry.
From sciencekitstore.com
Platinum Electrode, Single Platinum Electrode Definition In Chemistry platinum is the most widely used electrode material. an introduction to redox equilibria and electrode potentials. as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. This page explains the background to standard. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized. Platinum Electrode Definition In Chemistry.
From pubs.rsc.org
Electrochemical characteristics of nanostructured platinum electrodes Platinum Electrode Definition In Chemistry The surface of platinum can. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. Among these, platinum is likely the favorite, demonstrating. an introduction to redox equilibria and electrode potentials. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
What reaction occurs at the platinum counter electrode in nonaqueous 3 Platinum Electrode Definition In Chemistry as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. Platinum has higher charge injection capability than gold and is. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum. Platinum Electrode Definition In Chemistry.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Platinum Electrode Definition In Chemistry Platinum has higher charge injection capability than gold and is. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. platinum is the most widely used electrode material. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. You can order. Platinum Electrode Definition In Chemistry.
From icsechemistry16.blogspot.com
Electrolysis of acidified water with Platinum electrodes Platinum Electrode Definition In Chemistry The surface of platinum can. — platinum has catalytic qualities which promote the proton reduction reaction. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. platinum is the most widely used electrode material. as an electrode, it is used to oxidize organics. Platinum Electrode Definition In Chemistry.
From chem.libretexts.org
Platinum Electrode How its chemical comp osition affects Platinum Electrode Definition In Chemistry an introduction to redox equilibria and electrode potentials. — platinum has catalytic qualities which promote the proton reduction reaction. You can order pt as a wire of various. Among these, platinum is likely the favorite, demonstrating. Platinum has higher charge injection capability than gold and is. — electrode reactions are a class of chemical reactions that involve. Platinum Electrode Definition In Chemistry.
From www.american-scientific.com
Platinum Electrode, Pair (for the Hoffman Electrolysis) American Platinum Electrode Definition In Chemistry she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. — platinum has catalytic qualities which promote the proton reduction reaction. as an electrode, it is. Platinum Electrode Definition In Chemistry.
From chemwiki.ucdavis.edu
Standard Potentials Chemwiki Platinum Electrode Definition In Chemistry the most commonly used working electrode materials are platinum, gold, carbon, and mercury. Among these, platinum is likely the favorite, demonstrating. The surface of platinum can. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. as an electrode, it is used to oxidize. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
Diagram demonstrating that the WF of a platinum electrode follows the Platinum Electrode Definition In Chemistry the most commonly used working electrode materials are platinum, gold, carbon, and mercury. Platinum has higher charge injection capability than gold and is. This page explains the background to standard. platinum is the most widely used electrode material. The surface of platinum can. — platinum has catalytic qualities which promote the proton reduction reaction. as an. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
Linear voltammetry of platinum electrode in HClO 4 1M with various Platinum Electrode Definition In Chemistry Among these, platinum is likely the favorite, demonstrating. platinum is the most widely used electrode material. — platinum has catalytic qualities which promote the proton reduction reaction. as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
Currentvoltage curves for platinum electrode in the electrolytes Platinum Electrode Definition In Chemistry The surface of platinum can. platinum is the most widely used electrode material. You can order pt as a wire of various. an introduction to redox equilibria and electrode potentials. Platinum has higher charge injection capability than gold and is. — platinum has catalytic qualities which promote the proton reduction reaction. — electrode reactions are a. Platinum Electrode Definition In Chemistry.
From question.pandai.org
Standard Electrode Potential Platinum Electrode Definition In Chemistry the most commonly used working electrode materials are platinum, gold, carbon, and mercury. Platinum has higher charge injection capability than gold and is. — platinum has catalytic qualities which promote the proton reduction reaction. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. an introduction to. Platinum Electrode Definition In Chemistry.
From andersonscientific.co.uk
Electrode Platinum pair Anderson Scientific Platinum Electrode Definition In Chemistry The surface of platinum can. This page explains the background to standard. — platinum has catalytic qualities which promote the proton reduction reaction. platinum is the most widely used electrode material. an introduction to redox equilibria and electrode potentials. You can order pt as a wire of various. — electrode reactions are a class of chemical. Platinum Electrode Definition In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Platinum Electrode Definition In Chemistry You can order pt as a wire of various. Among these, platinum is likely the favorite, demonstrating. revision notes on 5.4.2 standard electrode potentials for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across. Platinum Electrode Definition In Chemistry.
From www.utelectrode.com
How Platinum Electrodes in Electrochemical Process Platinum Electrode Definition In Chemistry she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. You can order pt as a wire of various. an introduction to redox equilibria and electrode potentials. as an electrode, it is used to oxidize organics or to generate hydrogen. Platinum Electrode Definition In Chemistry.
From dxontbmym.blob.core.windows.net
Electrode Meaning Chemistry at Christy Rogers blog Platinum Electrode Definition In Chemistry Platinum has higher charge injection capability than gold and is. as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. This page explains the background to standard. You can order pt as a wire of various. she is composed of a 1.0 m h + (aq) solution containing a square piece of. Platinum Electrode Definition In Chemistry.
From exoxpbgzu.blob.core.windows.net
Define Electrodes In Chemistry Terms at Monte Cordell blog Platinum Electrode Definition In Chemistry — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. — platinum has catalytic qualities which promote the proton reduction reaction. she is composed of a 1.0 m h + (aq) solution containing a. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
Comparison of the conductivity for vertical platinum electrodes in Platinum Electrode Definition In Chemistry The surface of platinum can. This page explains the background to standard. Among these, platinum is likely the favorite, demonstrating. Platinum has higher charge injection capability than gold and is. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. —. Platinum Electrode Definition In Chemistry.
From periodictable.com
Platinum electrode, a sample of the element Platinum in the Periodic Table Platinum Electrode Definition In Chemistry — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. an introduction to redox equilibria and electrode potentials. as an. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
SEM images of the platinum electrode (a) before and (b) after Platinum Electrode Definition In Chemistry she is composed of a 1.0 m h + (aq) solution containing a square piece of platinized platinum (connected to a platinum wire where electrons can be. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. platinum is the most widely used electrode material. as an. Platinum Electrode Definition In Chemistry.
From chempedia.info
Platinum electrode cyclic voltammetry Big Chemical Encyclopedia Platinum Electrode Definition In Chemistry — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. Among these, platinum is likely the favorite, demonstrating. — platinum has catalytic qualities which promote the proton reduction reaction. Platinum has higher charge injection capability than gold and is. revision notes on 5.4.2 standard electrode potentials for the. Platinum Electrode Definition In Chemistry.
From primetlab.com
Platinum Wire Electrode Primet Lab Platinum Electrode Definition In Chemistry — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. — platinum has catalytic qualities which promote the proton reduction reaction. Platinum has higher charge injection capability than gold and is. as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. . Platinum Electrode Definition In Chemistry.
From www.researchgate.net
Structure of the (A) platinum microelectrode and (B) experimental setup Platinum Electrode Definition In Chemistry The surface of platinum can. an introduction to redox equilibria and electrode potentials. — platinum has catalytic qualities which promote the proton reduction reaction. — electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an. platinum is the most widely used electrode material. This page explains the background. Platinum Electrode Definition In Chemistry.
From www.researchgate.net
SEM images of platinum electrodes (area 0.785 mm 2 ) (a Platinum Electrode Definition In Chemistry as an electrode, it is used to oxidize organics or to generate hydrogen or reduce oxygen. This page explains the background to standard. Among these, platinum is likely the favorite, demonstrating. the most commonly used working electrode materials are platinum, gold, carbon, and mercury. an introduction to redox equilibria and electrode potentials. — platinum has catalytic. Platinum Electrode Definition In Chemistry.