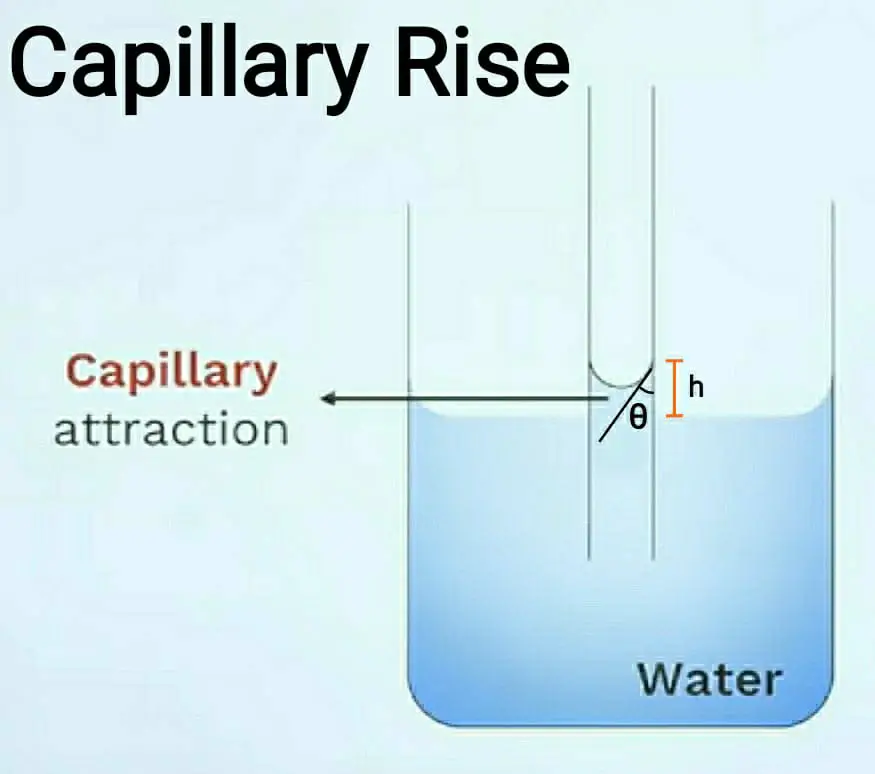
Capillary Tube Water Rise . However, liquids like mercury rise to a level that is lower than that of the liquid surrounding the tube. This phenomenon is called capillary action, and such tubes are called capillary tubes. if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding liquid level. when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. If is then very simple to calculate how far the liquid rises in terms of the surface tension, the angle of contact and the inside radius of the tube. This adhesion, together with surface tension in the water, produces an effect called capillarity, with a characteristic concave surface. A convex meniscus forms when molecules are more attracted to each other than they are to the container. capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and the tube’s surface. It is defined by the height to which the liquid climbs, counteracting the force of gravity. many liquids act like water and rise in a capillary tube. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube.
from dxotbmgjg.blob.core.windows.net
many liquids act like water and rise in a capillary tube. This phenomenon is called capillary action, and such tubes are called capillary tubes. if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding liquid level. However, liquids like mercury rise to a level that is lower than that of the liquid surrounding the tube. when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and the tube’s surface. This adhesion, together with surface tension in the water, produces an effect called capillarity, with a characteristic concave surface. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. It is defined by the height to which the liquid climbs, counteracting the force of gravity. A convex meniscus forms when molecules are more attracted to each other than they are to the container.
Surface Tension Of Water By Capillary Rise Method Pdf at Steven Helms blog
Capillary Tube Water Rise It is defined by the height to which the liquid climbs, counteracting the force of gravity. when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. This phenomenon is called capillary action, and such tubes are called capillary tubes. This adhesion, together with surface tension in the water, produces an effect called capillarity, with a characteristic concave surface. many liquids act like water and rise in a capillary tube. if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding liquid level. capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and the tube’s surface. However, liquids like mercury rise to a level that is lower than that of the liquid surrounding the tube. It is defined by the height to which the liquid climbs, counteracting the force of gravity. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. If is then very simple to calculate how far the liquid rises in terms of the surface tension, the angle of contact and the inside radius of the tube. A convex meniscus forms when molecules are more attracted to each other than they are to the container.
From byjus.com
61.Water rises in a capillary up to a height of 8.0cm .if the capillary Capillary Tube Water Rise If is then very simple to calculate how far the liquid rises in terms of the surface tension, the angle of contact and the inside radius of the tube. many liquids act like water and rise in a capillary tube. capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the. Capillary Tube Water Rise.
From bio.libretexts.org
2.16 Water Cohesive and Adhesive Properties Biology LibreTexts Capillary Tube Water Rise A convex meniscus forms when molecules are more attracted to each other than they are to the container. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. It is defined by the height to which the liquid climbs, counteracting the force of gravity. many liquids act like. Capillary Tube Water Rise.
From www.researchgate.net
Capillary rise in an inclined, cylindrical tube without evaporation Capillary Tube Water Rise when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. A convex meniscus forms when molecules are more attracted to each other than they are to the container. capillary rise is the phenomenon where a liquid ascends in a. Capillary Tube Water Rise.
From top10schoochurul.blogspot.com
What is Capillary Rise Method Capillary Tube Water Rise However, liquids like mercury rise to a level that is lower than that of the liquid surrounding the tube. if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding liquid level. when the lower end of a narrow capillary tube is immersed. Capillary Tube Water Rise.
From www.researchgate.net
Capillary rise dynamics and quantification. (A) Optical image of water Capillary Tube Water Rise capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and the tube’s surface. many liquids act like water and rise in a capillary tube. A convex meniscus forms when molecules are more attracted to each other than they. Capillary Tube Water Rise.
From www.slideserve.com
PPT Dynamics of Capillary Surfaces PowerPoint Presentation, free Capillary Tube Water Rise A convex meniscus forms when molecules are more attracted to each other than they are to the container. This phenomenon is called capillary action, and such tubes are called capillary tubes. If is then very simple to calculate how far the liquid rises in terms of the surface tension, the angle of contact and the inside radius of the tube.. Capillary Tube Water Rise.
From www.tutorix.com
To find the Surface Tension of Water by Capillary Rise Method Capillary Tube Water Rise many liquids act like water and rise in a capillary tube. if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding liquid level. This phenomenon is called capillary action, and such tubes are called capillary tubes. This adhesion, together with surface tension. Capillary Tube Water Rise.
From www.vedantu.com
When a capillary tube is immersed vertically in water class 11 physics Capillary Tube Water Rise water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. many liquids act like water and rise in a capillary tube. if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding. Capillary Tube Water Rise.
From infinitylearn.com
To Determine the Surface Tension of Water by Capillary Rise Method Capillary Tube Water Rise A convex meniscus forms when molecules are more attracted to each other than they are to the container. However, liquids like mercury rise to a level that is lower than that of the liquid surrounding the tube. It is defined by the height to which the liquid climbs, counteracting the force of gravity. if these narrow tubes are dipped. Capillary Tube Water Rise.
From www.youtube.com
Rise in liquid in Capillary Tube Surface Tension 12th Physics YouTube Capillary Tube Water Rise if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding liquid level. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. A convex meniscus forms when molecules are more attracted to. Capillary Tube Water Rise.
From www.youtube.com
Water rises in a vertical capillary tube upto 10 cm . If the tube is Capillary Tube Water Rise when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. It is defined by the height to which the liquid climbs, counteracting the force of gravity. water rises inside the capillary tube due to adhesion between water molecules and. Capillary Tube Water Rise.
From www.chegg.com
Solved CAPILLARY EFFECT • Capillary effect is the rise or Capillary Tube Water Rise This phenomenon is called capillary action, and such tubes are called capillary tubes. capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and the tube’s surface. It is defined by the height to which the liquid climbs, counteracting the. Capillary Tube Water Rise.
From dxotbmgjg.blob.core.windows.net
Surface Tension Of Water By Capillary Rise Method Pdf at Steven Helms blog Capillary Tube Water Rise This phenomenon is called capillary action, and such tubes are called capillary tubes. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. many liquids act like water and rise in a capillary tube. It is defined by the height to which the liquid climbs, counteracting the force. Capillary Tube Water Rise.
From www.researchgate.net
Rise of water in a capillary tube. On the right side the contact angle Capillary Tube Water Rise capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and the tube’s surface. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. If is then very simple. Capillary Tube Water Rise.
From sciencenotes.org
Capillary Action What It Is and How It Works Capillary Tube Water Rise It is defined by the height to which the liquid climbs, counteracting the force of gravity. A convex meniscus forms when molecules are more attracted to each other than they are to the container. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. many liquids act like. Capillary Tube Water Rise.
From www.sarthaks.com
Water rises to a height h in a capillary tube lowered vertically into Capillary Tube Water Rise A convex meniscus forms when molecules are more attracted to each other than they are to the container. This adhesion, together with surface tension in the water, produces an effect called capillarity, with a characteristic concave surface. It is defined by the height to which the liquid climbs, counteracting the force of gravity. water rises inside the capillary tube. Capillary Tube Water Rise.
From www.youtube.com
Water rises to a height `h` in a capillary tube of crosssectional area Capillary Tube Water Rise when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. It is defined by the height to which the liquid climbs, counteracting the force of gravity. water rises inside the capillary tube due to adhesion between water molecules and. Capillary Tube Water Rise.
From www.youtube.com
Surface tension of water by capillary rise method YouTube Capillary Tube Water Rise This phenomenon is called capillary action, and such tubes are called capillary tubes. many liquids act like water and rise in a capillary tube. It is defined by the height to which the liquid climbs, counteracting the force of gravity. If is then very simple to calculate how far the liquid rises in terms of the surface tension, the. Capillary Tube Water Rise.
From byjus.com
Determine The Surface Tension Of Water By Capillary Rise Method BYJU'S Capillary Tube Water Rise This phenomenon is called capillary action, and such tubes are called capillary tubes. If is then very simple to calculate how far the liquid rises in terms of the surface tension, the angle of contact and the inside radius of the tube. many liquids act like water and rise in a capillary tube. if these narrow tubes are. Capillary Tube Water Rise.
From byjus.com
a capillary tube of radius r immeresed in a liquid the liquid rises to Capillary Tube Water Rise This adhesion, together with surface tension in the water, produces an effect called capillarity, with a characteristic concave surface. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined. Capillary Tube Water Rise.
From www.researchgate.net
(a) Sketch of capillary rise in a vertical tube. (b) Sketch of the Capillary Tube Water Rise This phenomenon is called capillary action, and such tubes are called capillary tubes. A convex meniscus forms when molecules are more attracted to each other than they are to the container. However, liquids like mercury rise to a level that is lower than that of the liquid surrounding the tube. It is defined by the height to which the liquid. Capillary Tube Water Rise.
From www.researchgate.net
Mechanism of inner microstructures enhancing capillary rise in tubes Capillary Tube Water Rise A convex meniscus forms when molecules are more attracted to each other than they are to the container. many liquids act like water and rise in a capillary tube. water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. It is defined by the height to which the. Capillary Tube Water Rise.
From www.researchgate.net
Capillarydriven flow dynamics. (a) Schematic of capillarydriven flow Capillary Tube Water Rise when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. This phenomenon is called capillary action, and such tubes are called capillary tubes. if these narrow tubes are dipped in a liquid, it is observed that the liquid in. Capillary Tube Water Rise.
From web.mit.edu
Capillarity and gravity Capillary Tube Water Rise A convex meniscus forms when molecules are more attracted to each other than they are to the container. many liquids act like water and rise in a capillary tube. when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside.. Capillary Tube Water Rise.
From www.tutorix.com
To find the Surface Tension of Water by Capillary Rise Method Capillary Tube Water Rise This phenomenon is called capillary action, and such tubes are called capillary tubes. when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. if these narrow tubes are dipped in a liquid, it is observed that the liquid in. Capillary Tube Water Rise.
From www.researchgate.net
Capillary rise dynamics and quantification. (A) Optical image of water Capillary Tube Water Rise A convex meniscus forms when molecules are more attracted to each other than they are to the container. This adhesion, together with surface tension in the water, produces an effect called capillarity, with a characteristic concave surface. This phenomenon is called capillary action, and such tubes are called capillary tubes. It is defined by the height to which the liquid. Capillary Tube Water Rise.
From www.numerade.com
SOLVED If water rises in a capillary tube upto 3 cm. What is the Capillary Tube Water Rise It is defined by the height to which the liquid climbs, counteracting the force of gravity. This adhesion, together with surface tension in the water, produces an effect called capillarity, with a characteristic concave surface. many liquids act like water and rise in a capillary tube. if these narrow tubes are dipped in a liquid, it is observed. Capillary Tube Water Rise.
From www.ddcoatings.co.uk
What is Capillary Action? Capillary Tube Water Rise if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding liquid level. However, liquids like mercury rise to a level that is lower than that of the liquid surrounding the tube. many liquids act like water and rise in a capillary tube.. Capillary Tube Water Rise.
From www.mdpi.com
Applied Sciences Free FullText Effects of Tube Radius and Surface Capillary Tube Water Rise If is then very simple to calculate how far the liquid rises in terms of the surface tension, the angle of contact and the inside radius of the tube. It is defined by the height to which the liquid climbs, counteracting the force of gravity. This adhesion, together with surface tension in the water, produces an effect called capillarity, with. Capillary Tube Water Rise.
From exoqlqwqk.blob.core.windows.net
Surface Tension Of Water Using A Capillary Tube at Eddie Moss blog Capillary Tube Water Rise water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. This phenomenon is called capillary action, and such tubes are called capillary tubes. if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the. Capillary Tube Water Rise.
From dxotbmgjg.blob.core.windows.net
Surface Tension Of Water By Capillary Rise Method Pdf at Steven Helms blog Capillary Tube Water Rise if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the surrounding liquid level. capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and. Capillary Tube Water Rise.
From www.researchgate.net
Conceptual model for capillary rise and associated soilwater Capillary Tube Water Rise It is defined by the height to which the liquid climbs, counteracting the force of gravity. when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little above the level of the liquid outside. This phenomenon is called capillary action, and such tubes are called capillary tubes. If. Capillary Tube Water Rise.
From www.doubtnut.com
In a capillary tube, water rises to 3 mm . The height of water that wi Capillary Tube Water Rise water rises inside the capillary tube due to adhesion between water molecules and the glass walls of the capillary tube. It is defined by the height to which the liquid climbs, counteracting the force of gravity. when the lower end of a narrow capillary tube is immersed in a liquid, the liquid inside the tube rises a little. Capillary Tube Water Rise.
From dxotbmgjg.blob.core.windows.net
Surface Tension Of Water By Capillary Rise Method Pdf at Steven Helms blog Capillary Tube Water Rise capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and the tube’s surface. if these narrow tubes are dipped in a liquid, it is observed that the liquid in the capillary either rises or falls relative to the. Capillary Tube Water Rise.
From www.slideserve.com
PPT Chapter 10 INTRODUCTION AND PROPERTIES OF FLUIDS PowerPoint Capillary Tube Water Rise capillary rise is the phenomenon where a liquid ascends in a narrow tube or capillary due to the combined effect of surface tension and adhesive forces between the liquid and the tube’s surface. If is then very simple to calculate how far the liquid rises in terms of the surface tension, the angle of contact and the inside radius. Capillary Tube Water Rise.