Titration Lab Report Hcl And Naoh . To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m naoh solution and calculating their. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The initial volume and final volume of all three trails were subtracted to find each amount of. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Titrate with naoh solution till the first color change. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. You have to decide if this experiment is suitable to use with different classes, and look at the need for. In equation 1, the acid is hcl. Volume measurements play a key role in titration. This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh).
from www.chegg.com
This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). In equation 1, the acid is hcl. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. You have to decide if this experiment is suitable to use with different classes, and look at the need for. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Volume measurements play a key role in titration. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. The initial volume and final volume of all three trails were subtracted to find each amount of. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m naoh solution and calculating their. Titrate with naoh solution till the first color change.
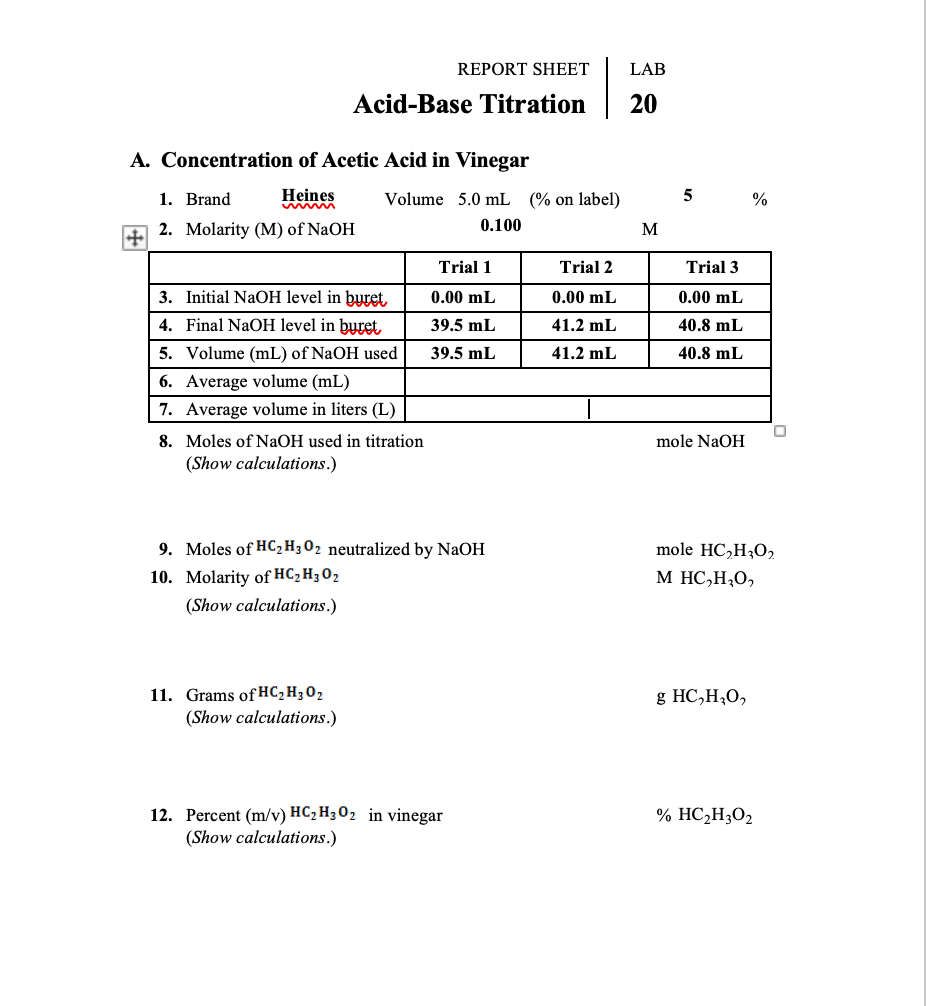
Solved REPORT SHEET LAB AcidBase Titration 20 A.
Titration Lab Report Hcl And Naoh Titrate with naoh solution till the first color change. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titrate with naoh solution till the first color change. In equation 1, the acid is hcl. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m naoh solution and calculating their. You have to decide if this experiment is suitable to use with different classes, and look at the need for. Volume measurements play a key role in titration. The initial volume and final volume of all three trails were subtracted to find each amount of.
From www.numerade.com
SOLVED Name Loxe Sco Lab Section Dale REPORT ON EXPERIMENT 8 Titration Lab Report Hcl And Naoh Titrate with naoh solution till the first color change. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. The initial volume and final volume of all three trails were subtracted to find each. Titration Lab Report Hcl And Naoh.
From www.slideshare.net
Titration Lab Report Titration Lab Report Hcl And Naoh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titrate with naoh solution till the first color change. The initial volume and final volume of all three trails were subtracted to find each amount of. To determine the precision of the titration of hcl with naoh by performing 4 trials. Titration Lab Report Hcl And Naoh.
From mungfali.com
Acid Base Titration Lab Titration Lab Report Hcl And Naoh Volume measurements play a key role in titration. This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). In equation 1, the acid is hcl. The initial volume and final volume of all three trails were subtracted to find each amount of. They then concentrate the solution. Titration Lab Report Hcl And Naoh.
From www.studypool.com
SOLUTION Chemistry lab report about titration between naoh and hcl Titration Lab Report Hcl And Naoh This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. One type of titration uses a neutralization reaction, in which an acid and a base react to produce. Titration Lab Report Hcl And Naoh.
From www.slideshare.net
Titration experiment report Titration Lab Report Hcl And Naoh Titrate with naoh solution till the first color change. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In equation 1, the acid is hcl. Result calculation according. Titration Lab Report Hcl And Naoh.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Titration Lab Report Hcl And Naoh The initial volume and final volume of all three trails were subtracted to find each amount of. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Volume measurements play a key role in titration. Titrate with naoh solution till the first color change. To determine the precision of the titration. Titration Lab Report Hcl And Naoh.
From studylib.net
Titration Lab Titration Lab Report Hcl And Naoh You have to decide if this experiment is suitable to use with different classes, and look at the need for. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m naoh solution and calculating their. The initial volume and final volume of all. Titration Lab Report Hcl And Naoh.
From www.studocu.com
Lab report Titration of HCl with NaOH Prelab Title Titration of Titration Lab Report Hcl And Naoh In equation 1, the acid is hcl. Titrate with naoh solution till the first color change. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To determine the. Titration Lab Report Hcl And Naoh.
From www.studeersnel.nl
Acid Base Lab lactic acid titration lab report Acid Base Titrations Titration Lab Report Hcl And Naoh Titrate with naoh solution till the first color change. This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to. Titration Lab Report Hcl And Naoh.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Titration Lab Report Hcl And Naoh Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. The initial volume and final volume of all three trails were subtracted to find each amount of. You have to decide if this experiment is suitable to use with different classes, and look at the need for. Volume. Titration Lab Report Hcl And Naoh.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Titration Lab Report Hcl And Naoh To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: You have to decide if this experiment is suitable to use with different classes, and look at the need for. Volume measurements play a. Titration Lab Report Hcl And Naoh.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Lab Report Hcl And Naoh One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: In equation 1, the acid is hcl. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Result calculation according to the reaction equation hcl + naoh → nacl. Titration Lab Report Hcl And Naoh.
From www.chegg.com
Solved LAB REPORT SHEET AcidBase Titration 12 . Titration Lab Report Hcl And Naoh Titrate with naoh solution till the first color change. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). Result calculation according to the reaction equation hcl + naoh → nacl +. Titration Lab Report Hcl And Naoh.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Titration Lab Report Hcl And Naoh You have to decide if this experiment is suitable to use with different classes, and look at the need for. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The initial volume and final volume of all three trails were subtracted to find each amount of. In equation 1, the acid is hcl. One. Titration Lab Report Hcl And Naoh.
From mungfali.com
Acid Base Titration Lab Titration Lab Report Hcl And Naoh In equation 1, the acid is hcl. Titrate with naoh solution till the first color change. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To determine the. Titration Lab Report Hcl And Naoh.
From www.chegg.com
Solved REPORT SHEET LAB AcidBase Titration 20 A. Titration Lab Report Hcl And Naoh Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. In equation 1, the acid. Titration Lab Report Hcl And Naoh.
From www.vrogue.co
Standardization Of Naoh Acid Base Titration Objective vrogue.co Titration Lab Report Hcl And Naoh They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The initial volume and final volume of all three trails were subtracted to find each amount of. Volume measurements play a key role in titration. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt. Titration Lab Report Hcl And Naoh.
From haileeqohicks.blogspot.com
Acid Base Titration Experiment Report HaileeqoHicks Titration Lab Report Hcl And Naoh In equation 1, the acid is hcl. Volume measurements play a key role in titration. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. This lab report shows the result and the. Titration Lab Report Hcl And Naoh.
From vdocuments.mx
Lab 26Date Titration of HCl Purpose Background Determine the molarity Titration Lab Report Hcl And Naoh In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Titrate with naoh solution till the first color change. The initial volume and final volume of all three trails were subtracted to find each amount of. Result. Titration Lab Report Hcl And Naoh.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Titration Lab Report Hcl And Naoh Titrate with naoh solution till the first color change. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. You have to decide if this experiment is suitable to. Titration Lab Report Hcl And Naoh.
From www.chegg.com
Solved Titration of HCl with standardized NaOH Titration Lab Report Hcl And Naoh They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. Volume measurements play a key role in titration. Titrate with naoh solution till the first color change. You have to decide if this experiment is suitable to use with different classes,. Titration Lab Report Hcl And Naoh.
From www.studocu.com
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH Titration Lab Report Hcl And Naoh This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m naoh solution and calculating their. Volume measurements play a key. Titration Lab Report Hcl And Naoh.
From studylib.net
Titration of Hydrochloric Acid with Sodium Hydroxide Titration Lab Report Hcl And Naoh This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Titrate with naoh solution till the first color change. To find the concentration of sodium. Titration Lab Report Hcl And Naoh.
From www.numerade.com
SOLVED The following data is from a conductometric titration of 10.00 Titration Lab Report Hcl And Naoh Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: The initial volume and final volume of all three trails were subtracted to find each amount. Titration Lab Report Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Lab Report Hcl And Naoh Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: To determine the precision of the titration of hcl with naoh by performing 4 trials of. Titration Lab Report Hcl And Naoh.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Titration Lab Report Hcl And Naoh They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. In equation 1, the acid is hcl. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. Titrate with naoh solution till the first color change. The initial volume and final volume of. Titration Lab Report Hcl And Naoh.
From www.studypool.com
SOLUTION Conductometric titration II lab report Studypool Titration Lab Report Hcl And Naoh This lab report shows the result and the analysis of the experiment of the titration of hydrochloric acid (hcl) with sodium hydroxide (naoh). One type of titration uses a neutralization reaction, in which an acid and a base react to produce a salt and water: Titrate with naoh solution till the first color change. To find the concentration of sodium. Titration Lab Report Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Titration Lab Report Hcl And Naoh Titrate with naoh solution till the first color change. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m naoh solution and calculating their. To find the concentration. Titration Lab Report Hcl And Naoh.
From www.studocu.com
Titration Report Titrating Acetic Acid and NaOH AcidBase Titration Titration Lab Report Hcl And Naoh The initial volume and final volume of all three trails were subtracted to find each amount of. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. To determine the precision of the titration of hcl with. Titration Lab Report Hcl And Naoh.
From www.chegg.com
Solved Section Titration Lab Report Sheet Table 1 Acid Base Titration Lab Report Hcl And Naoh To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m naoh solution and calculating their. The initial volume and final volume of all three trails were subtracted to find each amount of. To find the concentration of sodium hydroxide by titrating hydrochloric acid. Titration Lab Report Hcl And Naoh.
From kyrakruwesparza.blogspot.com
Acidbase Titration Lab Report Answers Hcl and Naoh KyrakruwEsparza Titration Lab Report Hcl And Naoh They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. Titrate with naoh solution till the first color change. In equation 1, the acid is hcl. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. To find the concentration of sodium hydroxide by titrating hydrochloric. Titration Lab Report Hcl And Naoh.
From www.numerade.com
SOLVED PART 2 CONDUCTOMETRIC TITRATION OF THE MIXTURE OF HCl AND Titration Lab Report Hcl And Naoh Titrate with naoh solution till the first color change. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. The initial volume and final volume of all three trails were subtracted to find each amount of. Volume measurements play a key role in titration. You have to decide. Titration Lab Report Hcl And Naoh.
From mungfali.com
Acid Base Titration Lab Titration Lab Report Hcl And Naoh The initial volume and final volume of all three trails were subtracted to find each amount of. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Volume measurements play a key role in titration. To determine the precision of the titration of hcl with naoh by performing 4 trials of. Titration Lab Report Hcl And Naoh.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Titration Lab Report Hcl And Naoh To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. You have to decide if this experiment is suitable to use with different classes, and look at the need for. Result calculation according to the reaction equation hcl + naoh → nacl + h 2 o hydrochloric acid reacts with sodium hydroxide. Titrate with naoh solution. Titration Lab Report Hcl And Naoh.
From childhealthpolicy.vumc.org
😱 Titration lab report discussion. Lab Report 9. 20221018 Titration Lab Report Hcl And Naoh To find the concentration of sodium hydroxide by titrating hydrochloric acid with sodium hydroxide. To determine the precision of the titration of hcl with naoh by performing 4 trials of titrating 5 ml samples of diluted acid with a 0 m naoh solution and calculating their. They then concentrate the solution and allow it to crystallise to produce sodium chloride. Titration Lab Report Hcl And Naoh.