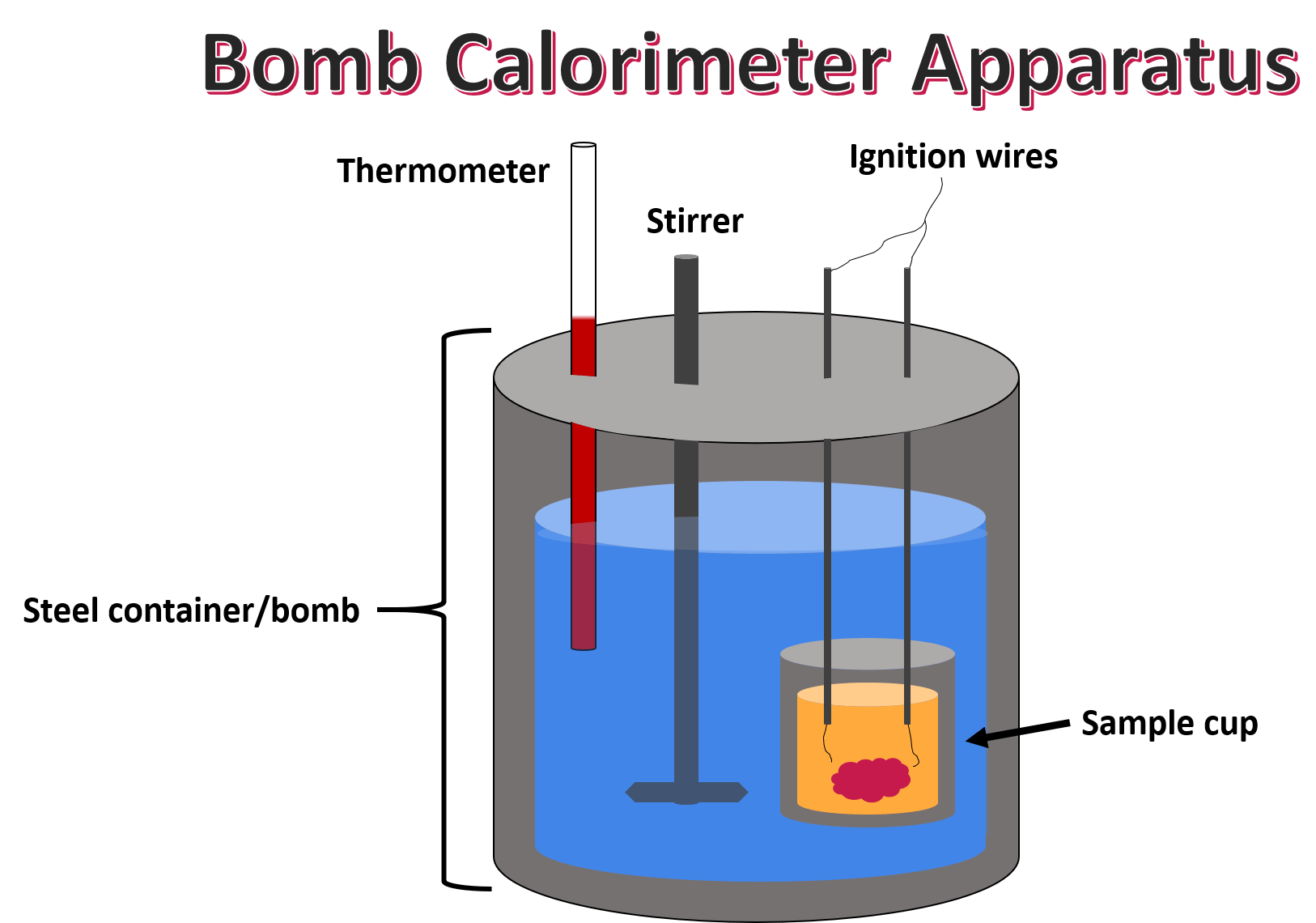
Calorimeter Bomb Experiment . You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. A tutorial guide on how to calculate the heat of combustion. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. This lab demonstrates one of the most common. One of the most important types of. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. Bomb calorimetry is a technique that allows you to directly measure the heat of.
from www.expii.com
The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. This lab demonstrates one of the most common. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. A tutorial guide on how to calculate the heat of combustion. You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. One of the most important types of. Bomb calorimetry is a technique that allows you to directly measure the heat of.
Bomb Calorimeter — Structure & Function Expii
Calorimeter Bomb Experiment A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. A tutorial guide on how to calculate the heat of combustion. Bomb calorimetry is a technique that allows you to directly measure the heat of. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. One of the most important types of. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter Bomb Experiment One of the most important types of. Bomb calorimetry is a technique that allows you to directly measure the heat of. A tutorial guide on how to calculate the heat of combustion. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. The purpose of this research is to determine. Calorimeter Bomb Experiment.
From wps.prenhall.com
Media Portfolio Calorimeter Bomb Experiment The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion. Calorimeter Bomb Experiment.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Calorimeter Bomb Experiment The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. One of the most important types of. Bomb calorimetry is a technique that allows you. Calorimeter Bomb Experiment.
From www.medicalexpo.com
Isothermal titration calorimeter C 1 1/10 IKA laboratory / compact Calorimeter Bomb Experiment One of the most important types of. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. This lab demonstrates one of the most common. Bomb calorimetry is a technique that allows you to directly measure the heat of. You will watch in lab a. Calorimeter Bomb Experiment.
From www.pinterest.com
Oxygen Bomb Calorimeters DDS CAOLRIMETERS Dds, Bombs, Oxygen Calorimeter Bomb Experiment To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. This lab demonstrates one of the most common. You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. One of the most important types of. The bomb calorimeter is. Calorimeter Bomb Experiment.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Calorimeter Bomb Experiment To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. A tutorial guide on how to calculate the heat of combustion. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. One of the. Calorimeter Bomb Experiment.
From chemistry.about.com
Coffee Cup and Bomb Calorimetry Calorimeter Bomb Experiment A tutorial guide on how to calculate the heat of combustion. One of the most important types of. Bomb calorimetry is a technique that allows you to directly measure the heat of. This lab demonstrates one of the most common. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific. Calorimeter Bomb Experiment.
From medium.com
The Essential Guide to Watch Glass in Laboratory Uses, Benefits, and Calorimeter Bomb Experiment The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat. Calorimeter Bomb Experiment.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter Bomb Experiment The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. This lab demonstrates one of the most common. You will watch. Calorimeter Bomb Experiment.
From www.chegg.com
A student runs two experiments with a constantvolume Calorimeter Bomb Experiment Bomb calorimetry is a technique that allows you to directly measure the heat of. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. The. Calorimeter Bomb Experiment.
From www.chegg.com
A student runs two experiments with a constantvolume Calorimeter Bomb Experiment A tutorial guide on how to calculate the heat of combustion. Bomb calorimetry is a technique that allows you to directly measure the heat of. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall. Calorimeter Bomb Experiment.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Calorimeter Bomb Experiment The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. You will watch in lab a video that carries out the combustion reactions of. Calorimeter Bomb Experiment.
From wingle.jp
😂 Bomb calorimeter experiment conclusion. Experiment 1 A3 Bomb Calorimeter Bomb Experiment One of the most important types of. Bomb calorimetry is a technique that allows you to directly measure the heat of. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. A tutorial guide on how to calculate the heat of combustion. The experiment is. Calorimeter Bomb Experiment.
From drivenheisenberg.blogspot.com
Which Diagram Is A Bomb Calorimeter Drivenheisenberg Calorimeter Bomb Experiment You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. The experiment is carried out in an insulated sealed vessel called a bomb which is. Calorimeter Bomb Experiment.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Calorimeter Bomb Experiment You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific. Calorimeter Bomb Experiment.
From www.chegg.com
A student runs two experiments with a constantvolume Calorimeter Bomb Experiment One of the most important types of. You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. To determine accurate heats of combustion, bomb calorimeters. Calorimeter Bomb Experiment.
From www.tes.com
Elsie Widdowson video worksheets differentiated x3 Teaching Resources Calorimeter Bomb Experiment Bomb calorimetry is a technique that allows you to directly measure the heat of. A tutorial guide on how to calculate the heat of combustion. You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s. Calorimeter Bomb Experiment.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimeter Bomb Experiment One of the most important types of. Bomb calorimetry is a technique that allows you to directly measure the heat of. This lab demonstrates one of the most common. A tutorial guide on how to calculate the heat of combustion. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures.. Calorimeter Bomb Experiment.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Bomb Experiment Bomb calorimetry is a technique that allows you to directly measure the heat of. One of the most important types of. This lab demonstrates one of the most common. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. The purpose of this research is to determine the effect. Calorimeter Bomb Experiment.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Bomb Experiment The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. This lab demonstrates one of the most common. Bomb calorimetry is a technique that allows you. Calorimeter Bomb Experiment.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Bomb Experiment The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. This lab demonstrates one of the most common. A tutorial guide on how to calculate. Calorimeter Bomb Experiment.
From www.indiamart.com
Bomb Calorimeter (Fully Automatic) at Rs 125000.00/piece Korattur Calorimeter Bomb Experiment The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. One of the most important types of. A tutorial guide on how to calculate the. Calorimeter Bomb Experiment.
From jumpstarterdiscount.blogspot.com
Which Diagram Is A Bomb Calorimeter Wiring Diagram Calorimeter Bomb Experiment Bomb calorimetry is a technique that allows you to directly measure the heat of. This lab demonstrates one of the most common. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. A tutorial guide on how to calculate the heat of combustion. To determine. Calorimeter Bomb Experiment.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Bomb Experiment The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. One of the most important types of. A tutorial guide on how to calculate the heat of combustion. Bomb calorimetry is a technique that allows you to directly measure the heat of. To determine accurate heats. Calorimeter Bomb Experiment.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Calorimeter Bomb Experiment The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. Bomb calorimetry is a technique that allows you to directly measure the heat of. You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. The experiment. Calorimeter Bomb Experiment.
From jawabanguru.github.io
Parr Adiabatic Bomb Calorimeter Adalah Calorimeter Bomb Experiment To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. Bomb calorimetry is a technique that allows you to directly measure the heat of. The purpose of this research. Calorimeter Bomb Experiment.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimeter Bomb Experiment A tutorial guide on how to calculate the heat of combustion. This lab demonstrates one of the most common. You will watch in lab a video that carries out the combustion reactions of seven hydrocarbons in a bomb calorimeter. One of the most important types of. To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their. Calorimeter Bomb Experiment.
From saylordotorg.github.io
Calorimetry Calorimeter Bomb Experiment A tutorial guide on how to calculate the heat of combustion. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs.. Calorimeter Bomb Experiment.
From www.indiamart.com
Bomb Calorimeters, बम कैलोरीमीटर in Bengaluru , Proxor Instruments And Calorimeter Bomb Experiment The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. The experiment is carried out in an insulated sealed vessel called a bomb which is designed to withstand high pressures. A tutorial guide on how to calculate the heat of combustion. One of the most important. Calorimeter Bomb Experiment.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Bomb Experiment To determine accurate heats of combustion, bomb calorimeters are typically calibrated to give their overall heat capacity of the specific ‘bomb. A tutorial guide on how to calculate the heat of combustion. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. Bomb calorimetry is. Calorimeter Bomb Experiment.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:3.1.2 Calorimetry Calorimeter Bomb Experiment The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. To determine accurate heats of combustion, bomb calorimeters are typically calibrated. Calorimeter Bomb Experiment.
From www.laboratory-equipment.com
Laboratory Calorimeters from IKA Calorimeter Bomb Experiment One of the most important types of. The bomb calorimeter is a laboratory instrument used to measure the amount of a sample’s combustion heat or heat power when excess oxygen combustion occurs. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. A tutorial guide on. Calorimeter Bomb Experiment.
From users.highland.edu
Calorimetry Calorimeter Bomb Experiment This lab demonstrates one of the most common. Bomb calorimetry is a technique that allows you to directly measure the heat of. The purpose of this research is to determine the effect of using the bomb calorimeter on the ability of physics students to process science. A tutorial guide on how to calculate the heat of combustion. The experiment is. Calorimeter Bomb Experiment.