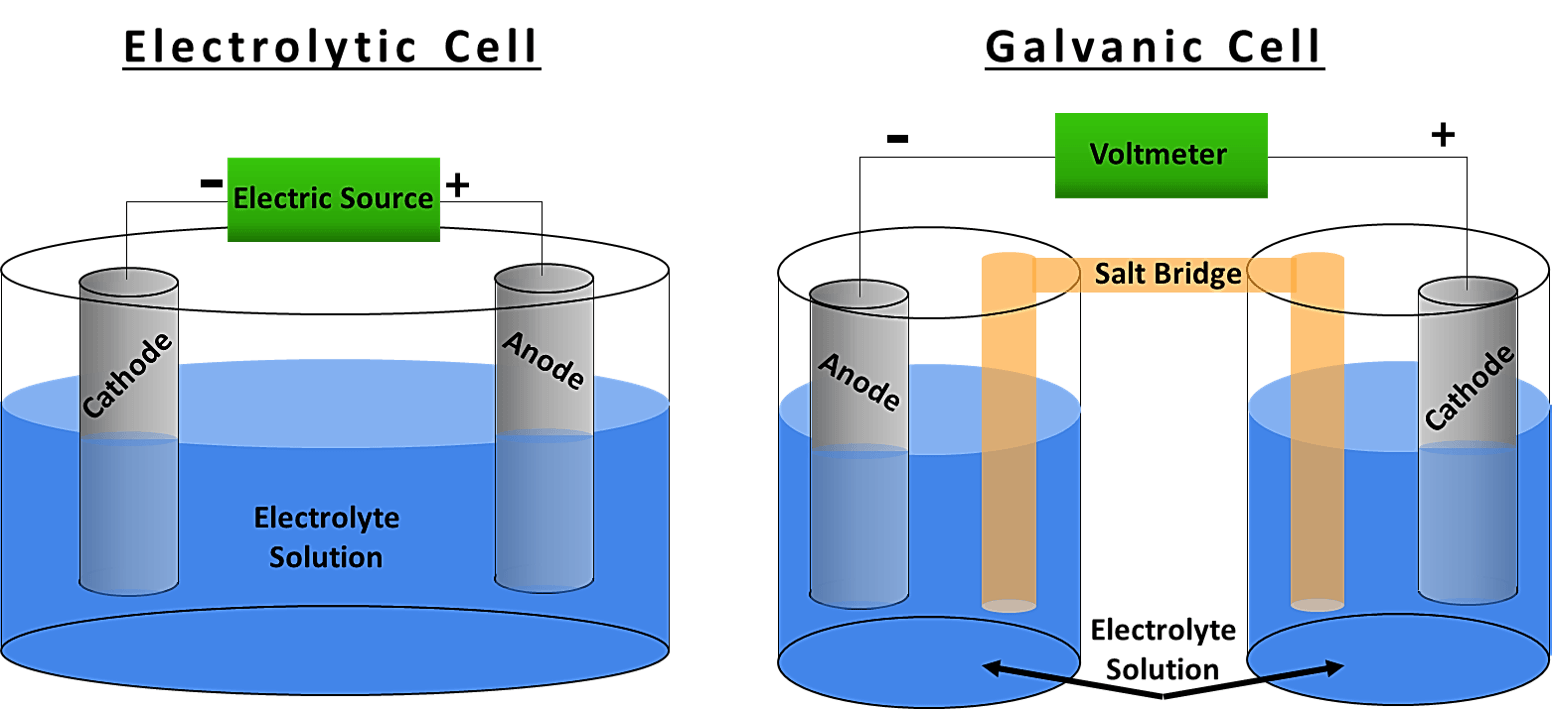
Electrochemical Vs Electrolytic Cell . In an electrochemical cell, half. However, there are also striking differences between the two cells. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In electrochemical cells, a spontaneous reaction occurs. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to.
from www.expii.com
An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However, there are also striking differences between the two cells. In an electrochemical cell, half. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. In electrochemical cells, a spontaneous reaction occurs. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell.
Electrochemical Cell — Definition & Overview Expii
Electrochemical Vs Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However, there are also striking differences between the two cells. In electrochemical cells, a spontaneous reaction occurs. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. In an electrochemical cell, half. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode.
From www.difference.wiki
Electrochemical Cell vs. Electrolytic Cell What’s the Difference? Electrochemical Vs Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. In an electrochemical cell, half. Electrolytic cells are very similar. Electrochemical Vs Electrolytic Cell.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. In electrochemical cells, a spontaneous reaction occurs. The most significant difference. Electrochemical Vs Electrolytic Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. In an electrochemical cell, half. Electrochemical cells and electrolytic cells are. Electrochemical Vs Electrolytic Cell.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. In an electrochemical cell, half. Electrochemical cells and electrolytic cells are. Electrochemical Vs Electrolytic Cell.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. In electrochemical cells, a spontaneous reaction occurs. In an electrochemical cell, half. However, there are also striking differences between the two. Electrochemical Vs Electrolytic Cell.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. In an electrochemical cell, half. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy. Electrochemical Vs Electrolytic Cell.
From www.youtube.com
The differences between the voltaic cell and the electrolytic cell Electrochemical Vs Electrolytic Cell However, there are also striking differences between the two cells. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In electrochemical cells, a spontaneous reaction occurs. Electrolytic cells are very similar to voltaic (galvanic). Electrochemical Vs Electrolytic Cell.
From www.pinterest.co.uk
What are the differences between Galvanic cell and Electrolytic cell Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. However, there are also. Electrochemical Vs Electrolytic Cell.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog Electrochemical Vs Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have. Electrochemical Vs Electrolytic Cell.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. An electrochemical cell splits the oxidant and reductant in a manner. Electrochemical Vs Electrolytic Cell.
From mavink.com
Electrochemical Cell Diagram Electrochemical Vs Electrolytic Cell In an electrochemical cell, half. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy. Electrochemical Vs Electrolytic Cell.
From slidetodoc.com
Electrochemistry Spontaneity of Redox Reactions 21 1 Electrochemistry Electrochemical Vs Electrolytic Cell However, there are also striking differences between the two cells. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge,. Electrochemical Vs Electrolytic Cell.
From www.toppr.com
I. Cathode is ve terminal both in electrochemical and electrolytic Electrochemical Vs Electrolytic Cell However, there are also striking differences between the two cells. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. The most significant difference between an electrolytic cell and an electrochemical. Electrochemical Vs Electrolytic Cell.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Electrochemical Vs Electrolytic Cell However, there are also striking differences between the two cells. In electrochemical cells, a spontaneous reaction occurs. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have. Electrochemical Vs Electrolytic Cell.
From exotyizys.blob.core.windows.net
What Is The Difference Between Electrochemical And Electrolytic Cell at Electrochemical Vs Electrolytic Cell Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. However, there are also striking differences between the two cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In electrochemical cells, a spontaneous reaction occurs. The most significant difference between an electrolytic cell and an. Electrochemical Vs Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells Electrochemical Vs Electrolytic Cell In electrochemical cells, a spontaneous reaction occurs. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. However, there are also striking differences between the two cells. Electrochemical cells and electrolytic cells are two fundamental devices used in. Electrochemical Vs Electrolytic Cell.
From stoplearn.com
Electrochemical Cells 2023 Electrochemical Vs Electrolytic Cell However, there are also striking differences between the two cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical.. Electrochemical Vs Electrolytic Cell.
From byjus.com
An electrolytic cell is used to convert Electrochemical Vs Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. Electrochemical Vs Electrolytic Cell.
From www.youtube.com
9.2 Comparison of electrochemical cells (SL) YouTube Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. However, there are. Electrochemical Vs Electrolytic Cell.
From stock.adobe.com
Vector scientific illustration of the electrolysis processes. Set of Electrochemical Vs Electrolytic Cell In electrochemical cells, a spontaneous reaction occurs. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. However, there are also striking differences between the two cells. In an electrochemical cell, half. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. Electrolytic cells are. Electrochemical Vs Electrolytic Cell.
From www.youtube.com
Electrochemical Cell vs Electrolytic Cell Electrochemistry YouTube Electrochemical Vs Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However, there are also striking differences between the two cells. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the. Electrochemical Vs Electrolytic Cell.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog Electrochemical Vs Electrolytic Cell In electrochemical cells, a spontaneous reaction occurs. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In an electrochemical cell, half. The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. However, there are also striking differences between the two cells. Electrolytic cells are very similar to. Electrochemical Vs Electrolytic Cell.
From narodnatribuna.info
Electrolysis Electrolytic Cells And Electrolysis Process Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. Electrochemical cells and electrolytic cells are two fundamental devices used in. Electrochemical Vs Electrolytic Cell.
From www.pinterest.pt
Galvanic Cell and Electrolytic Cell Electrochemical Cells Teaching Electrochemical Vs Electrolytic Cell However, there are also striking differences between the two cells. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. In an electrochemical cell, half. Electrochemical cells and electrolytic cells are. Electrochemical Vs Electrolytic Cell.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell Electrochemical Vs Electrolytic Cell In an electrochemical cell, half. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However,. Electrochemical Vs Electrolytic Cell.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell Electrochemical Vs Electrolytic Cell Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However, there are also striking differences between the two cells. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt. Electrochemical Vs Electrolytic Cell.
From www.studypool.com
SOLUTION Electrochemical cell vs electrolytic cell Studypool Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. In an electrochemical cell, half. However, there are also striking differences between the two cells. The most significant difference between an. Electrochemical Vs Electrolytic Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrochemical Vs Electrolytic Cell In an electrochemical cell, half. In electrochemical cells, a spontaneous reaction occurs. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However, there are also striking differences between the two cells. Electrolytic cells are very similar. Electrochemical Vs Electrolytic Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. In electrochemical cells, a spontaneous reaction occurs. The most significant difference between an electrolytic cell and an electrochemical cell is that. Electrochemical Vs Electrolytic Cell.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. In an electrochemical cell, half. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. However,. Electrochemical Vs Electrolytic Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Vs Electrolytic Cell Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. In an electrochemical cell, half. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy. Electrochemical Vs Electrolytic Cell.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID Electrochemical Vs Electrolytic Cell In an electrochemical cell, half. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both have a consistent flow of electrons from the anode to the cathode. However, there are also striking differences between the two cells. In electrochemical cells, a spontaneous reaction. Electrochemical Vs Electrolytic Cell.
From www.doubtnut.com
Electrochemical Vs Electrolytic Cell Electrochemical Vs Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. In an electrochemical cell, half. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have. Electrochemical Vs Electrolytic Cell.
From www.askdifference.com
Electrochemical Cell vs. Electrolytic Cell — What’s the Difference? Electrochemical Vs Electrolytic Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side, and both. Electrochemical Vs Electrolytic Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Electrochemical Vs Electrolytic Cell The most significant difference between an electrolytic cell and an electrochemical cell is that an electrolytic cell. In electrochemical cells, a spontaneous reaction occurs. Electrochemical cells and electrolytic cells are two fundamental devices used in electrochemistry to convert chemical energy into electrical. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge,. Electrochemical Vs Electrolytic Cell.