Piccolo Occluder . As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. Amplatzer duct occluders are flexible and. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants.
from www.revespcardiol.org
The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. Amplatzer duct occluders are flexible and. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent.
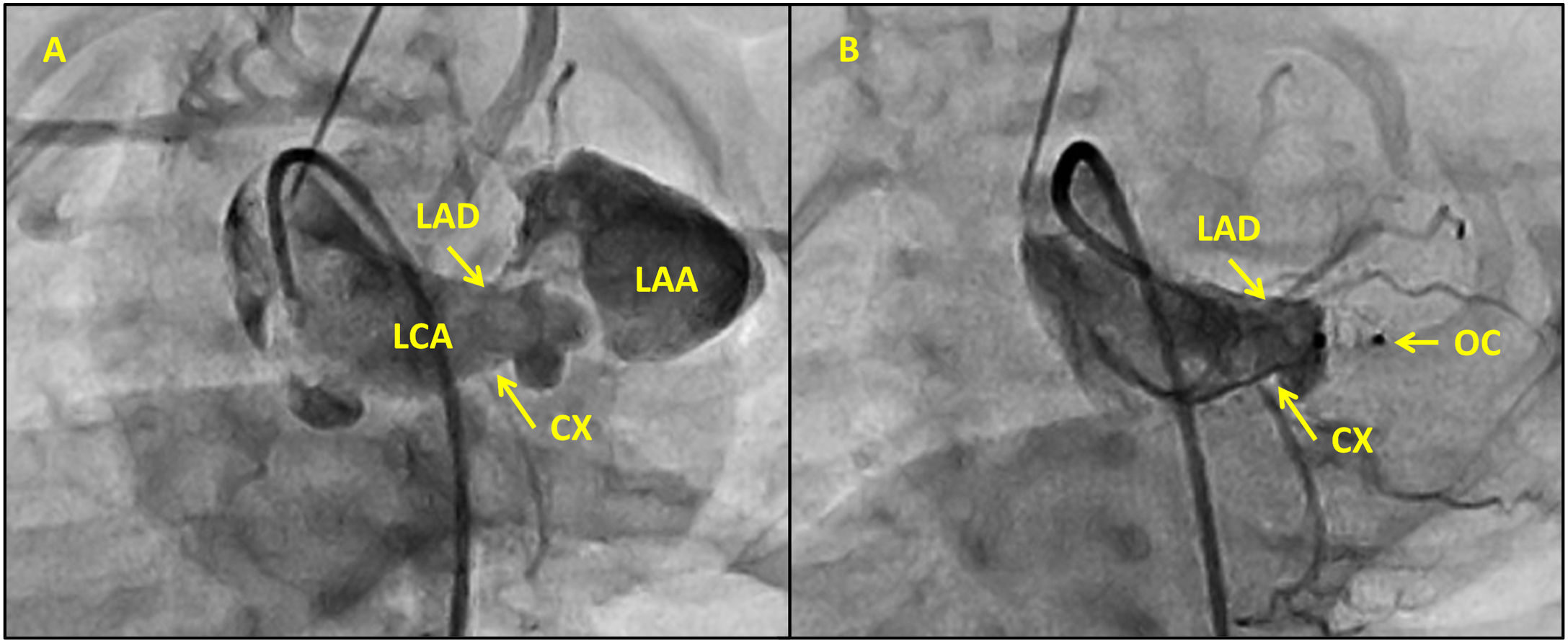
Closure of coronary artery fistula using Piccolo occluder Revista
Piccolo Occluder The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Amplatzer duct occluders are flexible and. Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to.
From www.bibamedtech.com
CE marks for Masters HP 15mm and Amplatzer Piccolo Occluder BIBA Piccolo Occluder The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo occluder. Piccolo Occluder.
From www.cambridge.org
Successful occlusion of a feeding artery with Amplatzer Piccolo Piccolo Occluder Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The amplatzer piccolo™ occluder, is designed. Piccolo Occluder.
From www.researchgate.net
(PDF) Percutaneous Closure of Patent Ductus Arteriosus with Amplatzer Piccolo Occluder The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Results reinforce that. Piccolo Occluder.
From www.researchgate.net
(PDF) Amplatzer Piccolo Occluder clinical trial for percutaneous Piccolo Occluder Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Amplatzer duct occluders are flexible and. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3. Piccolo Occluder.
From www.revespcardiol.org
Closure of coronary artery fistula using Piccolo occluder Revista Piccolo Occluder Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. Amplatzer duct occluders are flexible and. The amplatzer piccolo occluder was successfully. Piccolo Occluder.
From expomedical.ru
Amplatzer Piccolo Окклюдер кардиологический Abbott Medical Piccolo Occluder The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. Amplatzer duct occluders are flexible and. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. The amplatzer piccolo™. Piccolo Occluder.
From www.revespcardiol.org
Closure of coronary artery fistula using Piccolo occluder Revista Piccolo Occluder As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Results reinforce that amplatzer piccolo™ occluder is safe and. Piccolo Occluder.
From www.revespcardiol.org
Closure of coronary artery fistula using Piccolo occluder Revista Piccolo Occluder The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Amplatzer duct occluders are flexible and.. Piccolo Occluder.
From www.medicaldevice-network.com
Abbott's Amplatzer Piccolo Occluder for babies gets FDA approval Piccolo Occluder Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. As the only. Piccolo Occluder.
From www.rdworldonline.com
Amplatzer Piccolo Occluder Research & Development World Piccolo Occluder Amplatzer duct occluders are flexible and. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven. Piccolo Occluder.
From www.structuralheart.abbott
Amplatzer Piccolo Occluder Piccolo Occluder The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric.. Piccolo Occluder.
From corpmedical.cl
AMPLATZER™ PICCOLO™ Corpmedical Piccolo Occluder Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. Amplatzer duct occluders are flexible and. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. Results reinforce that amplatzer piccolo™ occluder. Piccolo Occluder.
From www.cambridge.org
Aortic migration of Amplatzer Piccolo™ ductal Occluder Cardiology in Piccolo Occluder Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder is. Piccolo Occluder.
From www.cambridge.org
Aortic migration of Amplatzer Piccolo™ ductal Occluder Cardiology in Piccolo Occluder The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. Results reinforce. Piccolo Occluder.
From www.medicaldevice-network.com
Amplatzer Piccolo Occluder Device for Patent Ductus Arteriosus, USA Piccolo Occluder The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. Characterize the safety. Piccolo Occluder.
From abcnews.go.com
FDA approves new closure device for heart defect in premature babies Piccolo Occluder The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. As. Piccolo Occluder.
From structuralheart.insidepractice.com.au
Patent Ductus with the Amplatzer Piccolo™ Occluder Inside Practice Piccolo Occluder As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder is the only minimally invasive. Piccolo Occluder.
From www.researchgate.net
(PDF) Percutaneous patent ductus arteriosus closure in extremely low Piccolo Occluder Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients. Piccolo Occluder.
From www.revespcardiol.org
Closure of coronary artery fistula using Piccolo occluder Revista Piccolo Occluder The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. Characterize the. Piccolo Occluder.
From www.researchgate.net
photographs of the 4 mm Amplatzer Piccolo Occluder of three different Piccolo Occluder The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The. Piccolo Occluder.
From www.my-connext.com
Content General Piccolo Occluder Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. Amplatzer duct occluders are flexible and. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda). Piccolo Occluder.
From www.medicaldevice-network.com
Amplatzer Piccolo Occluder Device for Patent Ductus Arteriosus, USA Piccolo Occluder Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. The. Piccolo Occluder.
From www.medicaltubingandextrusion.com
How Abbott engineered a catheterdelivered device for premature babies Piccolo Occluder As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients. Piccolo Occluder.
From www.slideshare.net
Piccolo Duct Occluder.pdf Piccolo Occluder The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. As the only pda closure solution. Piccolo Occluder.
From www.medicaldevice-network.com
Amplatzer Piccolo Occluder Device for Patent Ductus Arteriosus, USA Piccolo Occluder As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. The amplatzer piccolo™ occluder is the only minimally invasive. Piccolo Occluder.
From onlinelibrary.wiley.com
Amplatzer Piccolo Occluder clinical trial for percutaneous closure of Piccolo Occluder The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. Amplatzer duct occluders are flexible and. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days. Piccolo Occluder.
From www.structuralheart.abbott
Amplatzer Piccolo Occluder Piccolo Occluder Amplatzer duct occluders are flexible and. The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants.. Piccolo Occluder.
From medicaldialogues.in
Abbott launches Amplatzer Piccolo Occluder in India to treat congenital Piccolo Occluder Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to. Piccolo Occluder.
From www.uhhospitals.org
Martin Bocks MD Discusses use of Piccolo Occluder Device to Repair Piccolo Occluder The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo occluder was successfully implanted in 95.5% of all study patients, including 99% of patients ≤2 kg. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe. Piccolo Occluder.
From www.researchgate.net
(PDF) Transcatheter Closure of a Patent Ductus Arteriosus Using a Piccolo Occluder Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective. Piccolo Occluder.
From www.revespcardiol.org
Closure of coronary artery fistula using Piccolo occluder Revista Piccolo Occluder Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to. Piccolo Occluder.
From www.cureus.com
Cureus Transcatheter Closure of a Patent Ductus Arteriosus Using a Piccolo Occluder The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. Results reinforce that amplatzer piccolo™ occluder is safe and effective for closing an opening in the heart known as a patent. The amplatzer piccolo™ occluder,. Piccolo Occluder.
From abcnews.go.com
FDA approves new closure device for heart defect in premature babies Piccolo Occluder The amplatzer piccolo™ occluder is the only minimally invasive pda closure device that is fda approved for premature infants. Amplatzer duct occluders are flexible and. As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. The amplatzer piccolo™. Piccolo Occluder.
From www.slideshare.net
Piccolo Duct Occluder.pdf Piccolo Occluder As the only pda closure solution indicated for premature infants ≥ 700g + ≥ 3 days old and proven to deliver safe and effective closure, amplatzer piccolo™ occluder offers new opportunities to. Amplatzer duct occluders are flexible and. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. The amplatzer piccolo™. Piccolo Occluder.
From www.cambridge.org
Successful occlusion of a feeding artery with Amplatzer Piccolo Piccolo Occluder Characterize the safety and effectiveness of the amplatzer piccolo occluder for patent ductus arteriosus (pda) closure. The amplatzer piccolo™ occluder, is designed to occlude small ducts in patients 700g and up, including neonates and infants. Discover the amplatzer piccolo occluder from abbott, designed for safe and effective closure of patent ductus arteriosus (pda) in pediatric. The amplatzer piccolo occluder was. Piccolo Occluder.