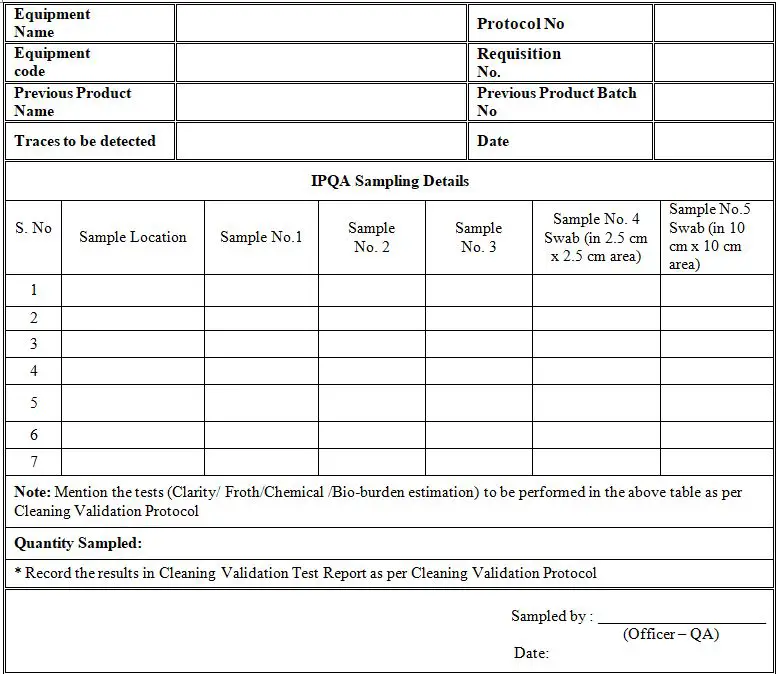
Cleaning Validation Plan Template . Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 6.1.1 on inspection, all surfaces must be visually. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. Us fda guide to inspection of validation of. A cleaning validation protocol outlines a plan of action. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or.
from pharmaguddu.com
A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. A cleaning validation protocol outlines a plan of action. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. 6.1.1 on inspection, all surfaces must be visually. Us fda guide to inspection of validation of. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of.
Cleaning Validation Protocol for Pharmaceutical Equipments » Pharmaguddu
Cleaning Validation Plan Template A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. A cleaning validation protocol outlines a plan of action. 6.1.1 on inspection, all surfaces must be visually. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. Us fda guide to inspection of validation of. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of.
From pharmagxp.com
Cleaning Validation The Definitive Guide Pharma GxP Cleaning Validation Plan Template Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. Us fda guide to inspection of validation of. 6.1.1 on inspection, all surfaces must be visually. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. A cleaning validation should provide documented evidence that when a defined cleaning. Cleaning Validation Plan Template.
From www.scribd.com
Template for Process Validation Protocol Verification And Validation Cleaning Validation Plan Template 6.1.1 on inspection, all surfaces must be visually. Us fda guide to inspection of validation of. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or.. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning ValidationSwab Test Sample PDF Accuracy And Precision Cleaning Validation Plan Template A cleaning validation protocol outlines a plan of action. 6.1.1 on inspection, all surfaces must be visually. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 12.7 cleaning validation • validation of cleaning procedures. Cleaning Validation Plan Template.
From pharmaguddu.com
Cleaning Validation Protocol for Pharmaceutical Equipments » Pharmaguddu Cleaning Validation Plan Template Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. A cleaning validation protocol outlines a plan of action. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product.. Cleaning Validation Plan Template.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Cleaning Validation Plan Template The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. 6.1.1 on inspection, all surfaces must be visually. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. A cleaning validation protocol outlines a plan of action.. Cleaning Validation Plan Template.
From www.egnyte.com
Validation Master Plan Egnyte Cleaning Validation Plan Template The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. 6.1.1 on inspection, all surfaces must be visually. Us fda guide to inspection of validation of. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Cleaning validation is a procedure. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning Validation Protocol Example Cleaning Validation Plan Template Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 6.1.1 on inspection, all surfaces must be visually. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. A cleaning validation protocol outlines a plan of action. The subject of cleaning validation in active. Cleaning Validation Plan Template.
From www.sampletemplates.com
FREE 9+ Sample Validation Plan Templates in PDF MS Word Cleaning Validation Plan Template Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. A cleaning validation protocol outlines a plan of action. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Us fda guide to inspection of validation of. 6.1.1 on inspection, all surfaces must be. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning Validation PICS Verification And Validation Calibration Cleaning Validation Plan Template Us fda guide to inspection of validation of. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. A cleaning validation protocol outlines a plan of action. 6.1.1 on inspection, all surfaces must be visually. A cleaning validation should provide documented evidence that when a defined cleaning process is used. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning Validation Master Plan Verification And Validation Cleaning Validation Plan Template Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. A cleaning validation protocol outlines a plan of action. A cleaning validation should provide documented evidence that when a defined cleaning process is used to. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning Validation Protocolexample.docx Verification And Validation Cleaning Validation Plan Template A cleaning validation protocol outlines a plan of action. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. Us fda guide to inspection of validation of. Cleaning validation is. Cleaning Validation Plan Template.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Cleaning Validation Plan Template A cleaning validation protocol outlines a plan of action. Us fda guide to inspection of validation of. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. A cleaning validation should provide documented evidence. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning Validation Protocol Purified Water Wellness Cleaning Validation Plan Template Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. 6.1.1 on inspection, all surfaces must be visually. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. Us fda guide to inspection of validation of. 12.7 cleaning validation • validation of cleaning procedures should. Cleaning Validation Plan Template.
From www.sampletemplates.com
FREE 9+ Sample Validation Plan Templates in PDF MS Word Cleaning Validation Plan Template 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. A cleaning validation protocol outlines a plan of action. Us fda guide to inspection of validation of. The subject of cleaning validation in active. Cleaning Validation Plan Template.
From ciqa.net
What is Cleaning Validation? • Download protocol templates Cleaning Validation Plan Template Us fda guide to inspection of validation of. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. A cleaning validation protocol outlines a plan of action. 12.7 cleaning validation. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning Validation Protocol PDF Verification And Validation Cleaning Validation Plan Template 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. Us fda guide to inspection of validation of. A cleaning validation should provide documented evidence that when a defined cleaning process is used to. Cleaning Validation Plan Template.
From sample-templates123.com
How To Create A Cleaning Estimate Free Sample, Example & Format Cleaning Validation Plan Template A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. A cleaning validation protocol. Cleaning Validation Plan Template.
From www.scribd.com
Sample Cleaning Validation Protocol Cleaning Validation Plan Template Us fda guide to inspection of validation of. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. A cleaning validation protocol outlines a plan of action. 6.1.1 on inspection, all surfaces must be visually. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs.. Cleaning Validation Plan Template.
From es.scribd.com
TEM260_Cleaning_Validation_Protocol_Template_sample.pdf Chemistry Cleaning Validation Plan Template Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or.. Cleaning Validation Plan Template.
From www.factssa.com
Infographic The difference between Allergen Cleaning Validation and Cleaning Validation Plan Template A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. Us fda guide to inspection of validation of. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has. Cleaning Validation Plan Template.
From studylib.net
Sample Cleaning Validation Protocol Cleaning Validation Plan Template A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. A cleaning validation protocol outlines a plan of action. Cleaning validation is a procedure of establishing. Cleaning Validation Plan Template.
From www.slideshare.net
Cleaning validation a complete know how Cleaning Validation Plan Template The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. 6.1.1 on inspection, all surfaces must be visually. A cleaning validation protocol outlines a plan of action.. Cleaning Validation Plan Template.
From sitemate.com
Post Construction Cleaning Checklist (Better than PDF template) Cleaning Validation Plan Template A cleaning validation protocol outlines a plan of action. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. Us fda guide to inspection of validation of. 6.1.1 on inspection, all surfaces must be visually. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants. Cleaning Validation Plan Template.
From www.vrogue.co
Cleaning Validation Protocol For Pharmaceutical Equip vrogue.co Cleaning Validation Plan Template Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. Cleaning validation is a procedure. Cleaning Validation Plan Template.
From doctemplates.us
12+ Cleaning Schedule Template For Office DocTemplates Cleaning Validation Plan Template A cleaning validation protocol outlines a plan of action. 6.1.1 on inspection, all surfaces must be visually. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. Us fda guide to inspection of validation of. Note, clean hold time can be established during evaluation of cleaning. Cleaning Validation Plan Template.
From www.sampletemplates.com
FREE 9+ Sample Validation Plan Templates in PDF MS Word Cleaning Validation Plan Template A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. Us fda guide to inspection of validation of. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. Note, clean hold time can be established during evaluation of cleaning performed. Cleaning Validation Plan Template.
From www.vrogue.co
Cleaning Validation Master Plan Verification And Vali vrogue.co Cleaning Validation Plan Template 6.1.1 on inspection, all surfaces must be visually. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if. Cleaning Validation Plan Template.
From pharmastate.academy
Cleaning Validation Protocol (CVP) Cleaning Validation Plan Template 6.1.1 on inspection, all surfaces must be visually. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning Validation Verification And Validation Sampling (Statistics) Cleaning Validation Plan Template Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. Us fda guide to inspection of validation of. 6.1.1. Cleaning Validation Plan Template.
From www.uslegalforms.com
Cleaning Validation Report Template Fill and Sign Printable Template Cleaning Validation Plan Template The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a large amount of. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. 6.1.1 on inspection, all surfaces. Cleaning Validation Plan Template.
From www.scribd.com
CLEANING PROCESSES AND CLEANING VALIDATION GUIDE Verification And Cleaning Validation Plan Template Us fda guide to inspection of validation of. 6.1.1 on inspection, all surfaces must be visually. A cleaning validation protocol outlines a plan of action. Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. A cleaning validation. Cleaning Validation Plan Template.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Cleaning Validation Plan Template A cleaning validation protocol outlines a plan of action. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. 6.1.1 on inspection, all surfaces must be visually. The subject of. Cleaning Validation Plan Template.
From safesitehq.com
Completing Your HACCP Plan Template a StepByStep Guide Safesite Cleaning Validation Plan Template Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. Us fda guide to inspection of validation of. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. The subject of cleaning validation in active pharmaceutical ingredient manufacturing plants has continued to receive a. Cleaning Validation Plan Template.
From www.scribd.com
Cleaning Validation Protocol Solution Verification And Validation Cleaning Validation Plan Template Us fda guide to inspection of validation of. A cleaning validation protocol outlines a plan of action. Note, clean hold time can be established during evaluation of cleaning performed on three validation runs. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis or. 6.1.1 on inspection, all surfaces must be. Cleaning Validation Plan Template.
From www.researchgate.net
(PDF) Cleaning Validation for the 21st Century Acceptance Limits for Cleaning Validation Plan Template Us fda guide to inspection of validation of. A cleaning validation should provide documented evidence that when a defined cleaning process is used to clean a machine, there is a high degree. A cleaning validation protocol outlines a plan of action. 12.7 cleaning validation • validation of cleaning procedures should reflect actual equipment usage patterns (12.71) • if various apis. Cleaning Validation Plan Template.