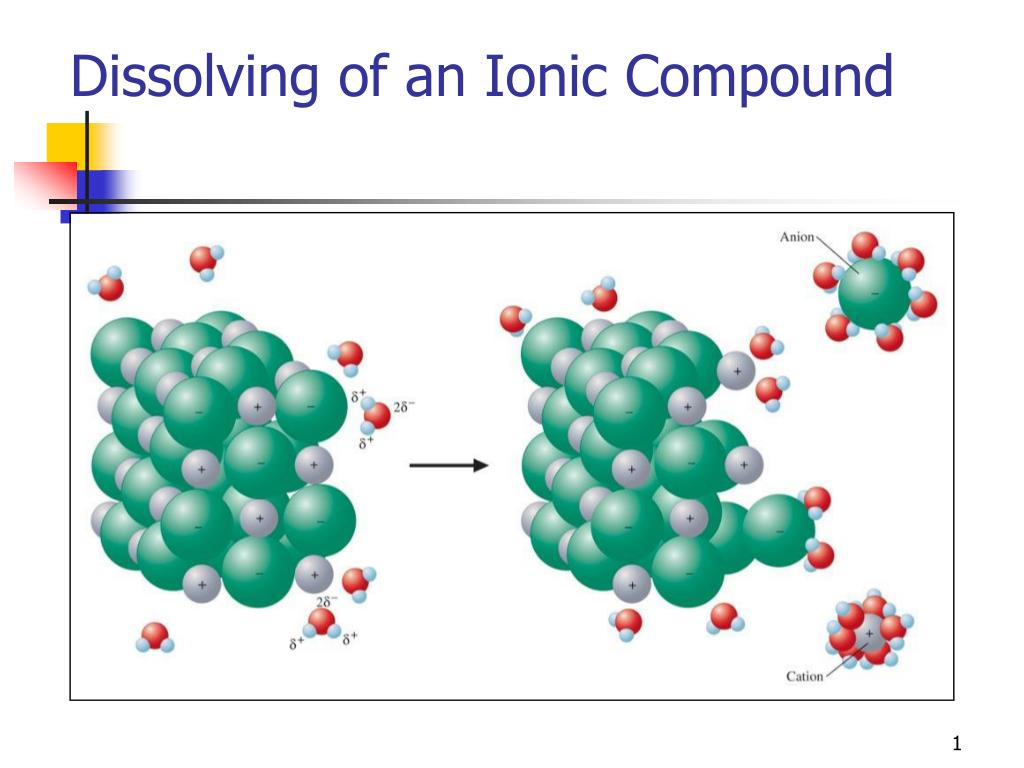
Why Do Ionic Crystal Dissolve In Water . this ionic compound dissolves readily in water. Nonpolar molecules such as those found in grease or oil do not dissolve in water. water typically dissolves many ionic compounds and polar molecules. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. during the reaction, the ionic bonds in sodium chloride are broken and replaced. Nonpolar molecules such as those found in grease. when ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. water typically dissolves many ionic compounds and polar molecules.
from www.slideserve.com
when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. when ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. during the reaction, the ionic bonds in sodium chloride are broken and replaced. Nonpolar molecules such as those found in grease. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. water typically dissolves many ionic compounds and polar molecules. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves.
PPT Dissolving of an Ionic Compound PowerPoint Presentation, free download ID3851009
Why Do Ionic Crystal Dissolve In Water this ionic compound dissolves readily in water. Nonpolar molecules such as those found in grease or oil do not dissolve in water. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. water typically dissolves many ionic compounds and polar molecules. this ionic compound dissolves readily in water. it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. Nonpolar molecules such as those found in grease. water typically dissolves many ionic compounds and polar molecules. during the reaction, the ionic bonds in sodium chloride are broken and replaced. when ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation.
From slideplayer.com
Properties of Solutions and Solubility ppt download Why Do Ionic Crystal Dissolve In Water Nonpolar molecules such as those found in grease. during the reaction, the ionic bonds in sodium chloride are broken and replaced. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. Nonpolar molecules such as those found in grease or oil do not dissolve in water. If the attraction between the ions. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 Why Do Ionic Crystal Dissolve In Water Nonpolar molecules such as those found in grease or oil do not dissolve in water. when ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound. Why Do Ionic Crystal Dissolve In Water.
From www.peoi.org
Chapter 4 Section C Ionic Equations A Closer Look Why Do Ionic Crystal Dissolve In Water Nonpolar molecules such as those found in grease. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. during the reaction, the ionic bonds in sodium chloride. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID1926480 Why Do Ionic Crystal Dissolve In Water If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Nonpolar molecules such as those found in grease. it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. this ionic compound dissolves readily. Why Do Ionic Crystal Dissolve In Water.
From en.ppt-online.org
Aqueous Solutions of Electrolytes online presentation Why Do Ionic Crystal Dissolve In Water Nonpolar molecules such as those found in grease or oil do not dissolve in water. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. water typically dissolves many ionic compounds and polar molecules. during the reaction, the ionic bonds in sodium chloride are. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation ID3205890 Why Do Ionic Crystal Dissolve In Water water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. this ionic compound dissolves readily in water. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. during the. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID2948816 Why Do Ionic Crystal Dissolve In Water water typically dissolves many ionic compounds and polar molecules. this ionic compound dissolves readily in water. Nonpolar molecules such as those found in grease. Nonpolar molecules such as those found in grease or oil do not dissolve in water. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. when. Why Do Ionic Crystal Dissolve In Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Visionlearning Why Do Ionic Crystal Dissolve In Water Nonpolar molecules such as those found in grease or oil do not dissolve in water. water typically dissolves many ionic compounds and polar molecules. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. during the reaction, the ionic bonds in sodium chloride are. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Dissolving of an Ionic Compound PowerPoint Presentation, free download ID3851009 Why Do Ionic Crystal Dissolve In Water If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. when ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. Nonpolar molecules such as those found in grease. this ionic compound dissolves readily. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download ID1926480 Why Do Ionic Crystal Dissolve In Water water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. this ionic compound dissolves readily in water. during the reaction, the ionic bonds in sodium chloride are broken and replaced. Nonpolar molecules such as those found in grease. when ionic compounds dissolve. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID3057625 Why Do Ionic Crystal Dissolve In Water during the reaction, the ionic bonds in sodium chloride are broken and replaced. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Nonpolar molecules such as those found in grease. when ionic compounds dissolve in water, they break apart into the ions that. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download ID9165094 Why Do Ionic Crystal Dissolve In Water when ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. Nonpolar molecules such as those found in grease or oil do not dissolve in water. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. it's not that ionic bonds. Why Do Ionic Crystal Dissolve In Water.
From www.youtube.com
Dissolution of Ionic Compounds YouTube Why Do Ionic Crystal Dissolve In Water when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. when ionic compounds dissolve in water, they break apart into the ions that make them up through a. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 Why Do Ionic Crystal Dissolve In Water this ionic compound dissolves readily in water. during the reaction, the ionic bonds in sodium chloride are broken and replaced. Nonpolar molecules such as those found in grease. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. when you immerse an ionic compound in water, the ions are attracted. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution Stoichiometry PowerPoint Why Do Ionic Crystal Dissolve In Water when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. during the reaction, the ionic bonds in sodium chloride are broken. Why Do Ionic Crystal Dissolve In Water.
From slideplayer.com
NOTES UNIT 5 WATER!. ppt download Why Do Ionic Crystal Dissolve In Water when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. during the reaction, the ionic bonds in sodium chloride are broken and replaced. when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. water typically dissolves many. Why Do Ionic Crystal Dissolve In Water.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Why Do Ionic Crystal Dissolve In Water when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. If the attraction between the ions and the water molecules is great enough to break the bonds holding the. Why Do Ionic Crystal Dissolve In Water.
From www.slideshare.net
Properties of Compounds Ionic, Covalent and Metallic Why Do Ionic Crystal Dissolve In Water If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. this ionic compound dissolves readily in water. Nonpolar molecules such as those found. Why Do Ionic Crystal Dissolve In Water.
From byjus.com
Madam Curie was studying solution chemistry. She was reading that dissolution happens with ionic Why Do Ionic Crystal Dissolve In Water when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. during the reaction, the ionic bonds in sodium chloride are broken and replaced. it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. water typically dissolves many ionic compounds. Why Do Ionic Crystal Dissolve In Water.
From www.sliderbase.com
Properties of Water Presentation Biology Why Do Ionic Crystal Dissolve In Water water typically dissolves many ionic compounds and polar molecules. during the reaction, the ionic bonds in sodium chloride are broken and replaced. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Nonpolar molecules such as those found in grease or oil do not. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download ID663487 Why Do Ionic Crystal Dissolve In Water it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. this ionic compound dissolves readily in water. water typically dissolves many ionic. Why Do Ionic Crystal Dissolve In Water.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube Why Do Ionic Crystal Dissolve In Water when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. this ionic compound dissolves readily in water. Nonpolar molecules such as those found in grease. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. when ionic. Why Do Ionic Crystal Dissolve In Water.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 Foundation Why Do Ionic Crystal Dissolve In Water during the reaction, the ionic bonds in sodium chloride are broken and replaced. water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease. water typically dissolves many ionic compounds and polar molecules. this ionic compound dissolves readily in water. it's not that ionic bonds are weaker than hydrogen. Why Do Ionic Crystal Dissolve In Water.
From www.youtube.com
Why do Ionic Solids Dissolve in Water (IonDipole IMF)? YouTube Why Do Ionic Crystal Dissolve In Water during the reaction, the ionic bonds in sodium chloride are broken and replaced. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. water typically dissolves many ionic compounds and polar molecules. it's not that ionic bonds are weaker than hydrogen bonds, but. Why Do Ionic Crystal Dissolve In Water.
From exohpqwmj.blob.core.windows.net
Which Crystals Dissolve In Water at Vivian Lamotte blog Why Do Ionic Crystal Dissolve In Water during the reaction, the ionic bonds in sodium chloride are broken and replaced. water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds. Why Do Ionic Crystal Dissolve In Water.
From byjus.com
Why ionic solids dissolve in water Why Do Ionic Crystal Dissolve In Water it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. this ionic compound dissolves readily in water. Nonpolar molecules such as those found in grease or oil do not dissolve in water. water typically dissolves many ionic compounds and polar molecules. If the attraction between the. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID1977818 Why Do Ionic Crystal Dissolve In Water it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. this ionic compound dissolves readily in water. water typically dissolves many ionic compounds and polar molecules. when ionic compounds dissolve in water, they break apart into the ions that make them up through a process. Why Do Ionic Crystal Dissolve In Water.
From www.bharatagritech.com
Ions In Aqueous Solution Infographic Diagram Showing, 54 OFF Why Do Ionic Crystal Dissolve In Water If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Nonpolar molecules such as those found in grease. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Nonpolar molecules such as. Why Do Ionic Crystal Dissolve In Water.
From webmis.highland.cc.il.us
Aqueous Solutions Why Do Ionic Crystal Dissolve In Water it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. this ionic compound dissolves readily in water. when you immerse an ionic compound in water, the ions are attracted to the water molecules, each of which carries a polar charge. Nonpolar molecules such as those found. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 Why Do Ionic Crystal Dissolve In Water it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. this ionic compound dissolves readily in water. water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease. water typically dissolves many ionic compounds and polar molecules. If the. Why Do Ionic Crystal Dissolve In Water.
From www.chegg.com
Solved Why do solid ionic compounds dissolve in water? A. Why Do Ionic Crystal Dissolve In Water Nonpolar molecules such as those found in grease or oil do not dissolve in water. when ionic compounds dissolve in water, they break apart into the ions that make them up through a process called dissociation. water typically dissolves many ionic compounds and polar molecules. when you immerse an ionic compound in water, the ions are attracted. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT The Chemical & Physical Basis of Life PowerPoint Presentation ID4223253 Why Do Ionic Crystal Dissolve In Water during the reaction, the ionic bonds in sodium chloride are broken and replaced. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. water typically dissolves many ionic compounds and polar molecules. when ionic compounds dissolve in water, they break apart into the. Why Do Ionic Crystal Dissolve In Water.
From courses.lumenlearning.com
Water Introduction to Biology Why Do Ionic Crystal Dissolve In Water it's not that ionic bonds are weaker than hydrogen bonds, but that the energy cost of breaking the ionic bonds is. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. when an ionic compound dissolves in water, the individual cations and anions are. Why Do Ionic Crystal Dissolve In Water.
From www.slideserve.com
PPT Ionic vs. Molecular Compounds PowerPoint Presentation ID3074480 Why Do Ionic Crystal Dissolve In Water when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. Nonpolar molecules such as those found in grease or oil do not dissolve in water. Nonpolar molecules such as those found in grease. water typically dissolves many ionic compounds and polar molecules. If the attraction between the ions and the water molecules. Why Do Ionic Crystal Dissolve In Water.
From getchemistry.weebly.com
Ionic Bonding Chemistry Why Do Ionic Crystal Dissolve In Water when an ionic compound dissolves in water, the individual cations and anions are completely surrounded by. during the reaction, the ionic bonds in sodium chloride are broken and replaced. If the attraction between the ions and the water molecules is great enough to break the bonds holding the ions together, the compound dissolves. Nonpolar molecules such as those. Why Do Ionic Crystal Dissolve In Water.