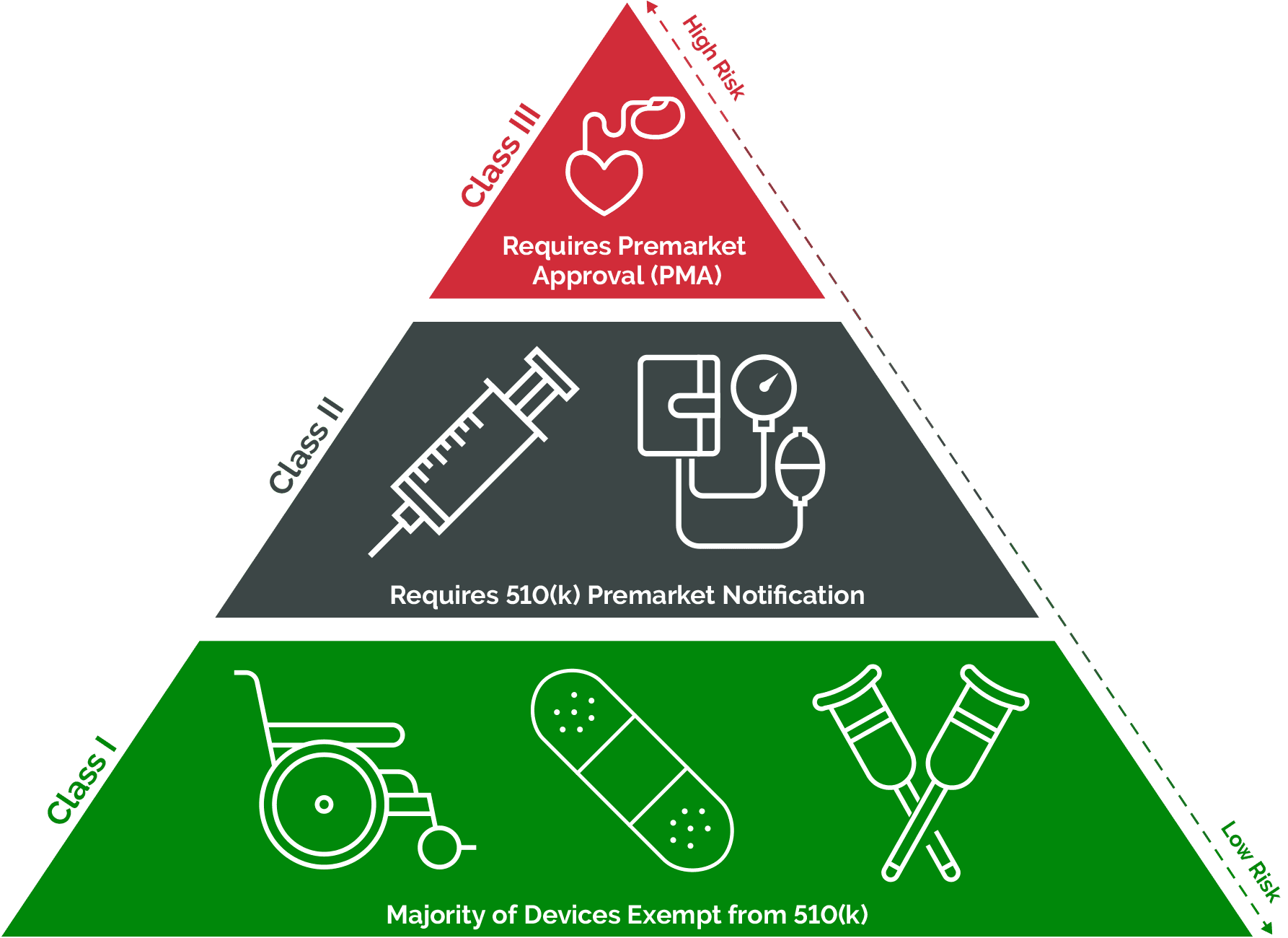
Medical Device Definition As Per Fda . Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The many medical devices on the market are not regulated as rigorously as medicinal products. Explain fda’s role in regulating medical devices. This report describes (1) fda’s authority to regulate medical devices; Describe five steps to get. Define a medical device and review basics about device classification. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. An instrument, apparatus, implement, machine,. (2) medical device classification and regulatory controls,.
from www.arenasolutions.com
Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The many medical devices on the market are not regulated as rigorously as medicinal products. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: This report describes (1) fda’s authority to regulate medical devices; Explain fda’s role in regulating medical devices. (2) medical device classification and regulatory controls,. Define a medical device and review basics about device classification. An instrument, apparatus, implement, machine,. Describe five steps to get. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,.
How to Classify Your Medical Device for FDA Approval Arena
Medical Device Definition As Per Fda Explain fda’s role in regulating medical devices. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Describe five steps to get. This report describes (1) fda’s authority to regulate medical devices; Explain fda’s role in regulating medical devices. (2) medical device classification and regulatory controls,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The many medical devices on the market are not regulated as rigorously as medicinal products. Define a medical device and review basics about device classification. An instrument, apparatus, implement, machine,.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Medical Device Definition As Per Fda Define a medical device and review basics about device classification. The many medical devices on the market are not regulated as rigorously as medicinal products. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. This report describes (1) fda’s authority to regulate medical devices; Explain fda’s. Medical Device Definition As Per Fda.
From gioixjpns.blob.core.windows.net
Device Definition Fda at Brian Hudgens blog Medical Device Definition As Per Fda Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Explain fda’s role in regulating medical devices. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. (2) medical device classification and regulatory controls,.. Medical Device Definition As Per Fda.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Device Definition As Per Fda An instrument, apparatus, implement, machine,. The many medical devices on the market are not regulated as rigorously as medicinal products. Explain fda’s role in regulating medical devices. This report describes (1) fda’s authority to regulate medical devices; (2) medical device classification and regulatory controls,. Define a medical device and review basics about device classification. As defined by 201 (h) of. Medical Device Definition As Per Fda.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Definition As Per Fda Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: This report describes (1) fda’s authority to regulate medical devices; An instrument, apparatus, implement, machine,. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. (2) medical device classification and regulatory controls,.. Medical Device Definition As Per Fda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Definition As Per Fda An instrument, apparatus, implement, machine,. Explain fda’s role in regulating medical devices. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. (2) medical device classification and regulatory controls,. Describe five steps to get. This report describes (1) fda’s authority to regulate medical devices; Section 201(h) of. Medical Device Definition As Per Fda.
From ramtechno.com
What Are the Three FDA Classes for Medical Devices? RAM Technologies Medical Device Definition As Per Fda This report describes (1) fda’s authority to regulate medical devices; An instrument, apparatus, implement, machine,. The many medical devices on the market are not regulated as rigorously as medicinal products. Explain fda’s role in regulating medical devices. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,.. Medical Device Definition As Per Fda.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Medical Device Definition As Per Fda Describe five steps to get. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement,. Medical Device Definition As Per Fda.
From www.presentationeze.com
FDA medical device classification PresentationEZE Medical Device Definition As Per Fda (2) medical device classification and regulatory controls,. This report describes (1) fda’s authority to regulate medical devices; As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. Explain fda’s role in regulating medical devices. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a. Medical Device Definition As Per Fda.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Device Definition As Per Fda The many medical devices on the market are not regulated as rigorously as medicinal products. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. Such a device will be classified by. Medical Device Definition As Per Fda.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Device Definition As Per Fda This report describes (1) fda’s authority to regulate medical devices; Define a medical device and review basics about device classification. An instrument, apparatus, implement, machine,. Describe five steps to get. The many medical devices on the market are not regulated as rigorously as medicinal products. Explain fda’s role in regulating medical devices. Such a device will be classified by regulation. Medical Device Definition As Per Fda.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Device Definition As Per Fda Describe five steps to get. An instrument, apparatus, implement, machine,. The many medical devices on the market are not regulated as rigorously as medicinal products. This report describes (1) fda’s authority to regulate medical devices; As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. Define a. Medical Device Definition As Per Fda.
From fyompphko.blob.core.windows.net
Medical Device Safety Alert Fda Definition at Sean Tyer blog Medical Device Definition As Per Fda (2) medical device classification and regulatory controls,. An instrument, apparatus, implement, machine,. The many medical devices on the market are not regulated as rigorously as medicinal products. Describe five steps to get. Define a medical device and review basics about device classification. Such a device will be classified by regulation into either class i (general controls), class ii (special controls). Medical Device Definition As Per Fda.
From exoyhcqns.blob.core.windows.net
Medical Device Definition Us Fda at James Grist blog Medical Device Definition As Per Fda The many medical devices on the market are not regulated as rigorously as medicinal products. Define a medical device and review basics about device classification. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant,. Medical Device Definition As Per Fda.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Definition As Per Fda Explain fda’s role in regulating medical devices. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. Define a medical device and review basics about device classification. Describe five steps to get. (2) medical device classification and regulatory controls,. An instrument, apparatus, implement, machine,. Such a device. Medical Device Definition As Per Fda.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Medical Device Definition As Per Fda An instrument, apparatus, implement, machine,. Define a medical device and review basics about device classification. The many medical devices on the market are not regulated as rigorously as medicinal products. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Explain fda’s role in regulating medical devices. Describe five steps to get. This report describes. Medical Device Definition As Per Fda.
From www.greenlight.guru
What is the FDA Medical Device Registration Process? Medical Device Definition As Per Fda The many medical devices on the market are not regulated as rigorously as medicinal products. (2) medical device classification and regulatory controls,. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. Such a device will be classified by regulation into either class i (general controls), class. Medical Device Definition As Per Fda.
From www.slideserve.com
PPT Risk Assessments Patient Safety and Innovation PowerPoint Medical Device Definition As Per Fda Describe five steps to get. Explain fda’s role in regulating medical devices. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Define a medical device and review basics about device classification. As defined by 201 (h) of the fd&c act, a medical device is an instrument,. Medical Device Definition As Per Fda.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Definition As Per Fda Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. (2) medical device classification and regulatory controls,. The many medical devices on the market are not regulated as rigorously as medicinal products. This report describes (1) fda’s authority to regulate medical devices; Section 201(h) of the food, drug & cosmetic act (fd&c act). Medical Device Definition As Per Fda.
From exoyhcqns.blob.core.windows.net
Medical Device Definition Us Fda at James Grist blog Medical Device Definition As Per Fda (2) medical device classification and regulatory controls,. An instrument, apparatus, implement, machine,. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Explain fda’s role in regulating medical devices. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine,. Medical Device Definition As Per Fda.
From premier-research.com
Medical Device Trials What You Need to Know About U.S. Regulations Medical Device Definition As Per Fda The many medical devices on the market are not regulated as rigorously as medicinal products. (2) medical device classification and regulatory controls,. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. This report describes (1) fda’s authority to regulate medical devices; As defined by 201 (h). Medical Device Definition As Per Fda.
From vem-medical.com
Medical Device Manufacturing Medical Device Definition As Per Fda Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. Explain fda’s role in regulating medical devices. The many medical devices on the market are not regulated as rigorously as medicinal products.. Medical Device Definition As Per Fda.
From www.swisstechnologiesne.com
Medical Devices and The FDA Medical Device Definition As Per Fda Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. (2) medical device classification and regulatory controls,. An instrument, apparatus, implement, machine,. Describe five steps to get. As defined by 201 (h) of the fd&c act, a medical. Medical Device Definition As Per Fda.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Definition As Per Fda The many medical devices on the market are not regulated as rigorously as medicinal products. This report describes (1) fda’s authority to regulate medical devices; As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. An instrument, apparatus, implement, machine,. Describe five steps to get. Such a. Medical Device Definition As Per Fda.
From www.slideserve.com
PPT FDA Regulation of In Vitro Diagnostic Tests PowerPoint Medical Device Definition As Per Fda Define a medical device and review basics about device classification. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Describe five steps to get. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: The many medical devices on the market. Medical Device Definition As Per Fda.
From www.slideserve.com
PPT FDA Medical Device Quality System Introduction PowerPoint Medical Device Definition As Per Fda Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Explain fda’s role in regulating medical devices. The many medical devices on the market are not regulated as rigorously as medicinal products. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),.. Medical Device Definition As Per Fda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Definition As Per Fda An instrument, apparatus, implement, machine,. (2) medical device classification and regulatory controls,. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: Describe five steps to get. Define a medical device and review basics about device classification. Such a device will be classified by regulation into either class i (general controls), class ii (special controls). Medical Device Definition As Per Fda.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Definition As Per Fda As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. An instrument, apparatus, implement, machine,. Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. Describe five steps to get. This report describes (1) fda’s authority to regulate medical devices;. Medical Device Definition As Per Fda.
From www.kolabtree.com
A guide to FDA Design Controls for your medical device Medical Device Definition As Per Fda This report describes (1) fda’s authority to regulate medical devices; Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Define a medical device and review basics about device classification. Explain fda’s role in regulating medical devices. Describe five steps to get. An instrument, apparatus, implement, machine,.. Medical Device Definition As Per Fda.
From www.pinterest.com
FDA approach to Medical Device Classification Medical device, Medical Medical Device Definition As Per Fda This report describes (1) fda’s authority to regulate medical devices; The many medical devices on the market are not regulated as rigorously as medicinal products. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines. Medical Device Definition As Per Fda.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Definition As Per Fda Describe five steps to get. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: (2) medical device classification and regulatory controls,. Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. The many medical devices on the market are not regulated as rigorously as medicinal products. As. Medical Device Definition As Per Fda.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Definition As Per Fda Describe five steps to get. (2) medical device classification and regulatory controls,. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. This report describes (1) fda’s authority to regulate medical devices; The many medical devices on the market are not regulated as rigorously as medicinal products.. Medical Device Definition As Per Fda.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Definition As Per Fda This report describes (1) fda’s authority to regulate medical devices; The many medical devices on the market are not regulated as rigorously as medicinal products. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. (2) medical device classification and regulatory controls,. As defined by 201 (h). Medical Device Definition As Per Fda.
From www.greenlight.guru
ISO 13485 and FDA QSR A StepbyStep Guide to Complying with Medical Medical Device Definition As Per Fda Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. Describe five steps to get. As defined by 201 (h) of the fd&c act, a medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent,. Define a medical device and review basics about device. Medical Device Definition As Per Fda.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Device Definition As Per Fda Explain fda’s role in regulating medical devices. Define a medical device and review basics about device classification. The many medical devices on the market are not regulated as rigorously as medicinal products. Describe five steps to get. Section 201(h) of the food, drug & cosmetic act (fd&c act) defines a device as: (2) medical device classification and regulatory controls,. Such. Medical Device Definition As Per Fda.
From medium.com
The 3 FDA medical device classes [differences and examples explained Medical Device Definition As Per Fda (2) medical device classification and regulatory controls,. This report describes (1) fda’s authority to regulate medical devices; An instrument, apparatus, implement, machine,. Such a device will be classified by regulation into either class i (general controls), class ii (special controls) or class iii (premarket approval),. The many medical devices on the market are not regulated as rigorously as medicinal products.. Medical Device Definition As Per Fda.