How To Take Boiling Point Of Liquid . If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. There are a variety of methods by which a sample's boiling point can be determined, including distillation, reflux, and by using a. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. This is the boiling point which is usually quoted in chemical literature. Measure and analyze the retention times and peak areas of toluene and. Produce a graph of time vs. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760 mm. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the.
from apollo.lsc.vsc.edu
The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Produce a graph of time vs. This is the boiling point which is usually quoted in chemical literature. There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760 mm. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. Normally when we boil a liquid, we do so at atmospheric pressure. Measure and analyze the retention times and peak areas of toluene and. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point.
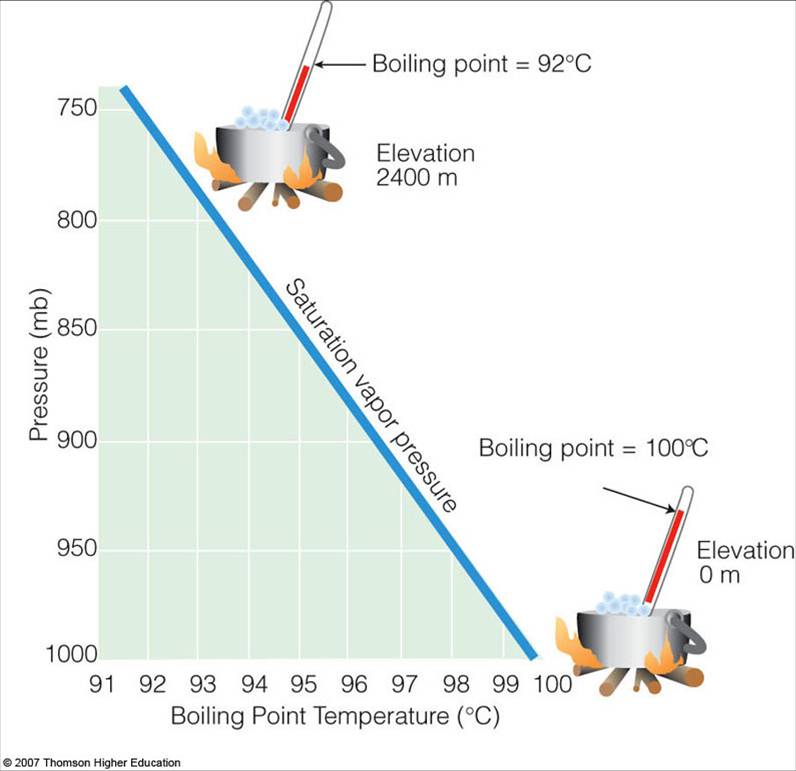
Saturation Vapor Pressure and the Boiling Point
How To Take Boiling Point Of Liquid Measure and analyze the retention times and peak areas of toluene and. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. There are a variety of methods by which a sample's boiling point can be determined, including distillation, reflux, and by using a. The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. This is the boiling point which is usually quoted in chemical literature. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Measure and analyze the retention times and peak areas of toluene and. Normally when we boil a liquid, we do so at atmospheric pressure. There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760 mm. Produce a graph of time vs.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps How To Take Boiling Point Of Liquid Measure and analyze the retention times and peak areas of toluene and. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. Produce a graph of time vs. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The procedure consists of. How To Take Boiling Point Of Liquid.
From www.coursehero.com
[Solved] The normal boiling point of a certain liquid X is 132.6°C How To Take Boiling Point Of Liquid There are a variety of methods by which a sample's boiling point can be determined, including distillation, reflux, and by using a. Normally when we boil a liquid, we do so at atmospheric pressure. This is the boiling point which is usually quoted in chemical literature. There are two ways in which a pressure nomograph can be used (i) to. How To Take Boiling Point Of Liquid.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts How To Take Boiling Point Of Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Normally when we boil a liquid, we do so at atmospheric pressure. There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760 mm. This is the boiling. How To Take Boiling Point Of Liquid.
From www.youtube.com
Boiling point diagram YouTube How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760 mm. The lower the pressure of a gas above. How To Take Boiling Point Of Liquid.
From byjus.com
Determination Of Boiling Point Of An Organic Compound Chemistry How To Take Boiling Point Of Liquid The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. There are a variety of methods by which a sample's boiling point can be determined, including distillation, reflux, and by using a. Produce a graph of time vs. The procedure consists of determining the temperature at which the external pressure on the. How To Take Boiling Point Of Liquid.
From www.youtube.com
Determine the boiling point of organic compounds YouTube How To Take Boiling Point Of Liquid The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. Measure and analyze the retention times and peak areas of toluene and. Produce a graph of time vs. The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in. How To Take Boiling Point Of Liquid.
From misael-blogzamora.blogspot.com
The Boiling Point of a Given Liquid Varies How To Take Boiling Point Of Liquid The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Produce a graph of time vs. A liquid boils at a temperature at which its. How To Take Boiling Point Of Liquid.
From www.youtube.com
Boiling Point from Heat of Vaporization (Example) YouTube How To Take Boiling Point Of Liquid The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760. How To Take Boiling Point Of Liquid.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube How To Take Boiling Point Of Liquid Normally when we boil a liquid, we do so at atmospheric pressure. The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. Produce a graph of time vs. The boiling point of a liquid depends on temperature, atmospheric pressure, and the. How To Take Boiling Point Of Liquid.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting How To Take Boiling Point Of Liquid If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. There are two ways in which a pressure nomograph can be used (i) to. How To Take Boiling Point Of Liquid.
From www.slideserve.com
PPT Ch. 13 States of Matter PowerPoint Presentation, free download How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. There are a variety of methods by which a sample's boiling point can be determined, including distillation, reflux, and by using a. Measure and analyze the retention times and peak areas. How To Take Boiling Point Of Liquid.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. Measure and analyze the retention times and peak areas of toluene and. This is the boiling point which is usually quoted in chemical literature. A liquid boils at a temperature at. How To Take Boiling Point Of Liquid.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox How To Take Boiling Point Of Liquid Measure and analyze the retention times and peak areas of toluene and. Normally when we boil a liquid, we do so at atmospheric pressure. The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. Produce a graph of time vs. This. How To Take Boiling Point Of Liquid.
From www.youtube.com
Measurement of boiling point of water practically YouTube How To Take Boiling Point Of Liquid Measure and analyze the retention times and peak areas of toluene and. There are a variety of methods by which a sample's boiling point can be determined, including distillation, reflux, and by using a. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. This is the boiling point which is usually quoted. How To Take Boiling Point Of Liquid.
From www.pinterest.com
Boiling Points for common Liquids and Gases Boiling point, Physical How To Take Boiling Point Of Liquid There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760 mm. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. Measure and analyze the retention times and. How To Take Boiling Point Of Liquid.
From www.youtube.com
BOILING POINT OF LIQUIDS YouTube How To Take Boiling Point Of Liquid Produce a graph of time vs. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. Normally when we boil a liquid, we do so at atmospheric pressure. There are a variety of methods by which a sample's boiling point can be determined, including distillation, reflux, and by using a. This is the. How To Take Boiling Point Of Liquid.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point How To Take Boiling Point Of Liquid Produce a graph of time vs. This is the boiling point which is usually quoted in chemical literature. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. There are a variety of methods by which a sample's boiling point can be. How To Take Boiling Point Of Liquid.
From blog.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. Produce a graph of time vs. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. This is the boiling point which is usually. How To Take Boiling Point Of Liquid.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. Measure and analyze the retention times and peak areas of toluene and. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas. How To Take Boiling Point Of Liquid.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy How To Take Boiling Point Of Liquid The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Produce a graph of time vs. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point which is usually. How To Take Boiling Point Of Liquid.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Produce a graph of time vs. There are a variety of methods by. How To Take Boiling Point Of Liquid.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps How To Take Boiling Point Of Liquid This is the boiling point which is usually quoted in chemical literature. There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760 mm. Normally when we boil a liquid, we do so at atmospheric pressure. A liquid boils at a temperature at which its vapor pressure is equal. How To Take Boiling Point Of Liquid.
From www.youtube.com
How To Draw Determination Of Boiling Point Of Water Diagram Easily Step How To Take Boiling Point Of Liquid Produce a graph of time vs. The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. This is the boiling point which is usually quoted in chemical literature. There are two ways in which a pressure nomograph can be used (i). How To Take Boiling Point Of Liquid.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. Measure and analyze the retention times and peak areas of toluene and. There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at. How To Take Boiling Point Of Liquid.
From sciencenotes.org
How to Boil Water at Room Temperature How To Take Boiling Point Of Liquid If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. Produce a graph of time vs. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. This is the boiling point which is usually. How To Take Boiling Point Of Liquid.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. Produce a graph of time vs. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its. How To Take Boiling Point Of Liquid.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps How To Take Boiling Point Of Liquid The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. This is the boiling point which is usually quoted in chemical literature. Normally when we boil a liquid, we do so at. How To Take Boiling Point Of Liquid.
From www.worksheetsplanet.com
What is Boiling Point How To Take Boiling Point Of Liquid The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Produce a graph of time vs. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Measure and analyze the retention times and peak areas of toluene and. The boiling point. How To Take Boiling Point Of Liquid.
From thepiquelab.com
Tackling Heat Energy Questions Melting & Boiling Points! Primary How To Take Boiling Point Of Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. This is the boiling point which is usually quoted in chemical literature. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling. How To Take Boiling Point Of Liquid.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube How To Take Boiling Point Of Liquid Measure and analyze the retention times and peak areas of toluene and. Normally when we boil a liquid, we do so at atmospheric pressure. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The lower the pressure of a gas above a liquid, the lower the temperature at. How To Take Boiling Point Of Liquid.
From www.haikudeck.com
13.2 by Carley Baker How To Take Boiling Point Of Liquid The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. There are a variety of methods by which a sample's boiling point can be determined, including distillation, reflux, and by using a. Measure and analyze the retention times and peak areas of toluene and. There are two ways in which a pressure nomograph. How To Take Boiling Point Of Liquid.
From www.youtube.com
CHEM 201 Calculating Boiling Point of a NonElectrolyte Solution YouTube How To Take Boiling Point Of Liquid The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. Measure and analyze the retention times and peak areas of toluene and. There are a variety of methods by which a sample's boiling point can. How To Take Boiling Point Of Liquid.
From jsmithmoore.com
Boiling point of ethanol celsius How To Take Boiling Point Of Liquid There are two ways in which a pressure nomograph can be used (i) to determine the boiling point at atmospheric pressure (760 mm. Produce a graph of time vs. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Measure and analyze the retention times and peak areas of. How To Take Boiling Point Of Liquid.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID How To Take Boiling Point Of Liquid Normally when we boil a liquid, we do so at atmospheric pressure. Measure and analyze the retention times and peak areas of toluene and. The lower the pressure of a gas above a liquid, the lower the temperature at which the liquid. This is the boiling point which is usually quoted in chemical literature. The procedure consists of determining the. How To Take Boiling Point Of Liquid.
From www.youtube.com
Boiling Point of Liquids YouTube How To Take Boiling Point Of Liquid The procedure consists of determining the temperature at which the external pressure on the boiling liquid is large enough to overcome the vapor pressure in a capillary tube inserted. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the. Measure and analyze the retention times and peak areas of toluene and. If this. How To Take Boiling Point Of Liquid.