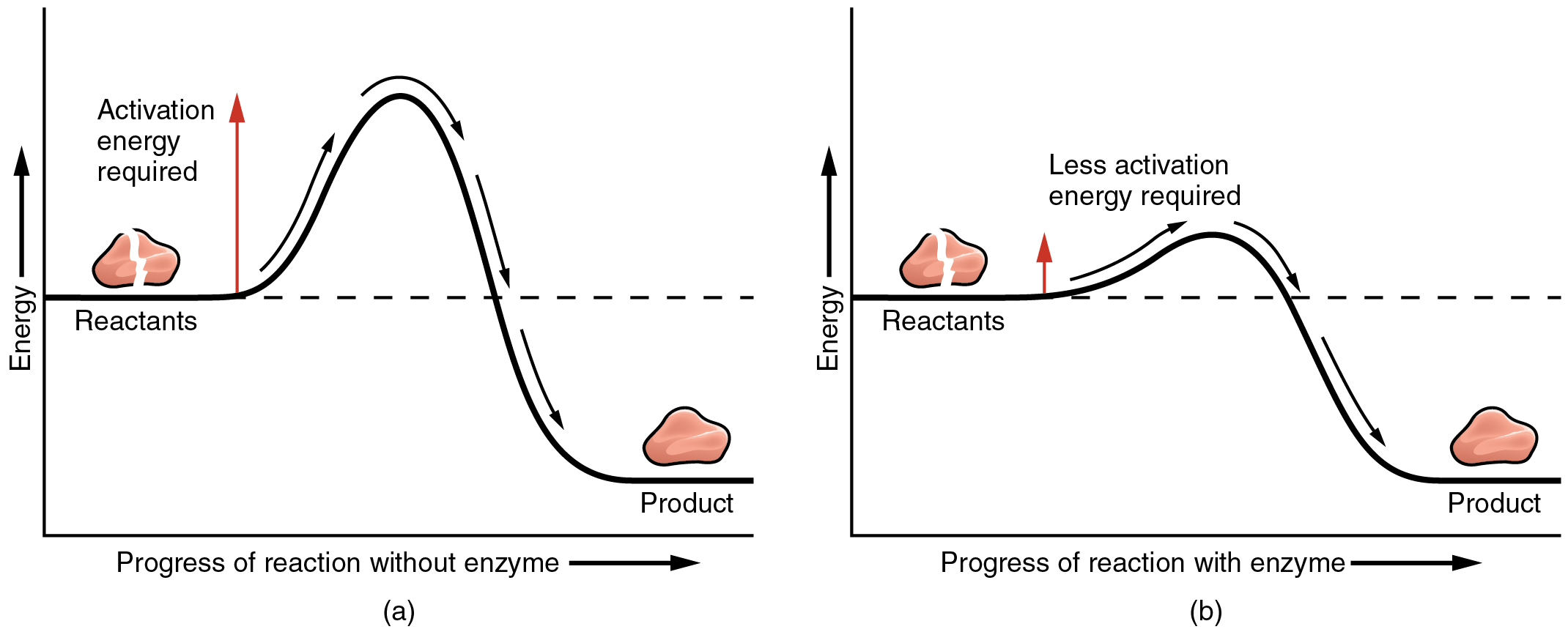
Enzymes Decrease The Rate Of Chemical Reactions . enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. enzymes (and other catalysts) act by reducing the activation energy,. They speed up the rate of chemical. enzymes allow chemical reactions to occur fast enough to support life. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. To some extent, this rule holds for all enzymatic reactions.
from giodveqaq.blob.core.windows.net
a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. They speed up the rate of chemical. enzymes (and other catalysts) act by reducing the activation energy,. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. enzymes allow chemical reactions to occur fast enough to support life. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. To some extent, this rule holds for all enzymatic reactions. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering.
What Do Enzymes Do In A Chemical Reaction at Richard Holmes blog
Enzymes Decrease The Rate Of Chemical Reactions a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. enzymes (and other catalysts) act by reducing the activation energy,. enzymes allow chemical reactions to occur fast enough to support life. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. They speed up the rate of chemical. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. To some extent, this rule holds for all enzymatic reactions.
From www.slideserve.com
PPT Enzyme Rate Enhancement PowerPoint Presentation, free download Enzymes Decrease The Rate Of Chemical Reactions a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. They speed up the rate of chemical. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Chapter 5 Enzymes PowerPoint Presentation, free download ID Enzymes Decrease The Rate Of Chemical Reactions enzymes allow chemical reactions to occur fast enough to support life. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. To some extent, this rule holds for all enzymatic reactions. After a certain point, however, an increase in temperature causes a decrease in the reaction rate,. Enzymes Decrease The Rate Of Chemical Reactions.
From www.genome.gov
Enzyme Enzymes Decrease The Rate Of Chemical Reactions enzymes allow chemical reactions to occur fast enough to support life. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. They speed up the rate of chemical. enzymes (and other catalysts) act by reducing the activation energy,. a general rule of thumb for most chemical reactions is that a temperature rise of. Enzymes Decrease The Rate Of Chemical Reactions.
From www.onlinebiologynotes.com
Rate of enzyme reactions and factor affecting the rate of enzyme Enzymes Decrease The Rate Of Chemical Reactions almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. To some extent, this rule holds for all enzymatic reactions. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. After a certain point, however, an increase in temperature causes a. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT EnZyMeS PowerPoint Presentation, free download ID2065739 Enzymes Decrease The Rate Of Chemical Reactions After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. enzymes allow chemical reactions to occur fast enough to support life. To some extent, this rule holds for all enzymatic reactions. a general rule of thumb for most chemical reactions is that a temperature. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Enzymes Decrease The Rate Of Chemical Reactions a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. They speed up the rate of chemical. a general rule of thumb for most chemical reactions is that a temperature. Enzymes Decrease The Rate Of Chemical Reactions.
From slidetodoc.com
Rates of Reactions and Enzymes Rates of Reaction Enzymes Decrease The Rate Of Chemical Reactions enzymes allow chemical reactions to occur fast enough to support life. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. enzymes (and other catalysts) act by reducing the activation energy,. After. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation ID2483456 Enzymes Decrease The Rate Of Chemical Reactions After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. enzymes (and other catalysts) act by reducing the activation energy,. almost all. Enzymes Decrease The Rate Of Chemical Reactions.
From www.expii.com
Enzymes Rate of Reaction Expii Enzymes Decrease The Rate Of Chemical Reactions To some extent, this rule holds for all enzymatic reactions. They speed up the rate of chemical. enzymes allow chemical reactions to occur fast enough to support life. enzymes (and other catalysts) act by reducing the activation energy,. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. almost all enzymes are proteins,. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideshare.net
Enzymes Enzymes Decrease The Rate Of Chemical Reactions a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. enzymes allow chemical reactions to occur fast enough to support life. enzymes (and other catalysts) act by reducing. Enzymes Decrease The Rate Of Chemical Reactions.
From giodveqaq.blob.core.windows.net
What Do Enzymes Do In A Chemical Reaction at Richard Holmes blog Enzymes Decrease The Rate Of Chemical Reactions After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. enzymes (and other catalysts) act by reducing the activation energy,. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. enzymes allow chemical reactions to occur fast enough to support. Enzymes Decrease The Rate Of Chemical Reactions.
From chemistrytalk.org
Enzymes Function and Types ChemTalk Enzymes Decrease The Rate Of Chemical Reactions They speed up the rate of chemical. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. To some extent, this rule holds for all enzymatic reactions. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. enzymes allow chemical reactions to occur fast enough to support. Enzymes Decrease The Rate Of Chemical Reactions.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Enzymes Decrease The Rate Of Chemical Reactions To some extent, this rule holds for all enzymatic reactions. They speed up the rate of chemical. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. enzymes (and other catalysts) act by reducing. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT HOW ENZYMES WORK PowerPoint Presentation, free download ID6954410 Enzymes Decrease The Rate Of Chemical Reactions After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. enzymes (and other catalysts) act by reducing the activation energy,. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. enzymes are chemical catalysts that accelerate. Enzymes Decrease The Rate Of Chemical Reactions.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii Enzymes Decrease The Rate Of Chemical Reactions enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. enzymes allow chemical reactions to occur fast enough to support life. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. To some extent, this rule holds for all enzymatic reactions.. Enzymes Decrease The Rate Of Chemical Reactions.
From www.geeksforgeeks.org
How Do Enzymes Bring About Such High Rates of Chemical Conversions Enzymes Decrease The Rate Of Chemical Reactions To some extent, this rule holds for all enzymatic reactions. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. They speed up the rate of chemical. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. . Enzymes Decrease The Rate Of Chemical Reactions.
From exyerwjum.blob.core.windows.net
How Do Enzymes Reduce Activation Energy at Pamela Edwards blog Enzymes Decrease The Rate Of Chemical Reactions After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. enzymes allow chemical reactions to occur fast enough to support life. They speed up the rate of chemical. enzymes (and other catalysts) act by reducing the activation energy,. almost all enzymes are proteins,. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Chapter 6 Enzyme PowerPoint Presentation, free download ID5692414 Enzymes Decrease The Rate Of Chemical Reactions enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. almost all enzymes. Enzymes Decrease The Rate Of Chemical Reactions.
From studymind.co.uk
Enzymes Rates of Reaction (Alevel Biology) Study Mind Enzymes Decrease The Rate Of Chemical Reactions They speed up the rate of chemical. enzymes allow chemical reactions to occur fast enough to support life. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. almost all. Enzymes Decrease The Rate Of Chemical Reactions.
From studymind.co.uk
Enzymes Rates of Reaction (Alevel Biology) Study Mind Enzymes Decrease The Rate Of Chemical Reactions almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. a general. Enzymes Decrease The Rate Of Chemical Reactions.
From www.expii.com
Concentration (Enzyme Reaction Rates) — Effects & Role Expii Enzymes Decrease The Rate Of Chemical Reactions enzymes allow chemical reactions to occur fast enough to support life. To some extent, this rule holds for all enzymatic reactions. They speed up the rate of chemical. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. enzymes are chemical catalysts that accelerate chemical reactions. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Chemical Reactions & Enzymes PowerPoint Presentation, free Enzymes Decrease The Rate Of Chemical Reactions enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. To some extent,. Enzymes Decrease The Rate Of Chemical Reactions.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Enzymes Decrease The Rate Of Chemical Reactions a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due. Enzymes Decrease The Rate Of Chemical Reactions.
From gioaralqv.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction In Such A Manner Enzymes Decrease The Rate Of Chemical Reactions enzymes (and other catalysts) act by reducing the activation energy,. To some extent, this rule holds for all enzymatic reactions. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. They speed up the rate of chemical. enzymes allow chemical reactions to occur fast. Enzymes Decrease The Rate Of Chemical Reactions.
From stock.adobe.com
Science infographic diagram show factors affecting enzyme activity Enzymes Decrease The Rate Of Chemical Reactions enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. To some extent, this rule holds for all enzymatic reactions. They speed up the rate of chemical. enzymes (and other catalysts) act by. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Chapter 5 Enzymes PowerPoint Presentation, free download ID Enzymes Decrease The Rate Of Chemical Reactions To some extent, this rule holds for all enzymatic reactions. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. They speed up the rate of chemical. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. enzymes allow chemical reactions to occur fast. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT ENZYMES PowerPoint Presentation, free download ID6886656 Enzymes Decrease The Rate Of Chemical Reactions almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. They speed up the rate of chemical. To some extent, this rule holds for all enzymatic reactions. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. enzymes are chemical. Enzymes Decrease The Rate Of Chemical Reactions.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Enzymes Decrease The Rate Of Chemical Reactions almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by lowering. After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. . Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID4283724 Enzymes Decrease The Rate Of Chemical Reactions After a certain point, however, an increase in temperature causes a decrease in the reaction rate, due to denaturation of the protein structure and. They speed up the rate of chemical. To some extent, this rule holds for all enzymatic reactions. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles. Enzymes Decrease The Rate Of Chemical Reactions.
From exyaxpzoa.blob.core.windows.net
Enzymes Decrease The Activation Energy Of A Reaction By at Deborah Enzymes Decrease The Rate Of Chemical Reactions They speed up the rate of chemical. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. enzymes (and other catalysts) act by reducing the activation energy,. a general. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 Enzymes Decrease The Rate Of Chemical Reactions They speed up the rate of chemical. enzymes allow chemical reactions to occur fast enough to support life. To some extent, this rule holds for all enzymatic reactions. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. enzymes are chemical catalysts that accelerate chemical reactions at physiological temperatures by. Enzymes Decrease The Rate Of Chemical Reactions.
From quizlet.com
effect of enzymes on chemical reaction rate Diagram Quizlet Enzymes Decrease The Rate Of Chemical Reactions almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. enzymes (and other catalysts) act by reducing the activation energy,. After a certain point, however, an increase in temperature causes. Enzymes Decrease The Rate Of Chemical Reactions.
From circuitwiringmoyle99.z22.web.core.windows.net
Parts Of An Enzyme Substrate Complex Enzymes Decrease The Rate Of Chemical Reactions almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. enzymes allow chemical reactions to occur fast enough to support life. To some extent, this rule holds for all enzymatic. Enzymes Decrease The Rate Of Chemical Reactions.
From www.slideserve.com
PPT Chemical reactions and enzymes PowerPoint Presentation, free Enzymes Decrease The Rate Of Chemical Reactions a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately. a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. enzymes (and other catalysts) act by reducing the activation energy,. They speed up the rate of chemical. enzymes. Enzymes Decrease The Rate Of Chemical Reactions.
From worksheetlistsog.z21.web.core.windows.net
Chemical Reactions And Enzymes Chart Enzymes Decrease The Rate Of Chemical Reactions a general rule of thumb for most chemical reactions is that a temperature rise of 10°c approximately doubles the reaction rate. almost all enzymes are proteins, comprised of amino acid chains, and they perform the critical task of lowering the activation. enzymes allow chemical reactions to occur fast enough to support life. enzymes are chemical catalysts. Enzymes Decrease The Rate Of Chemical Reactions.