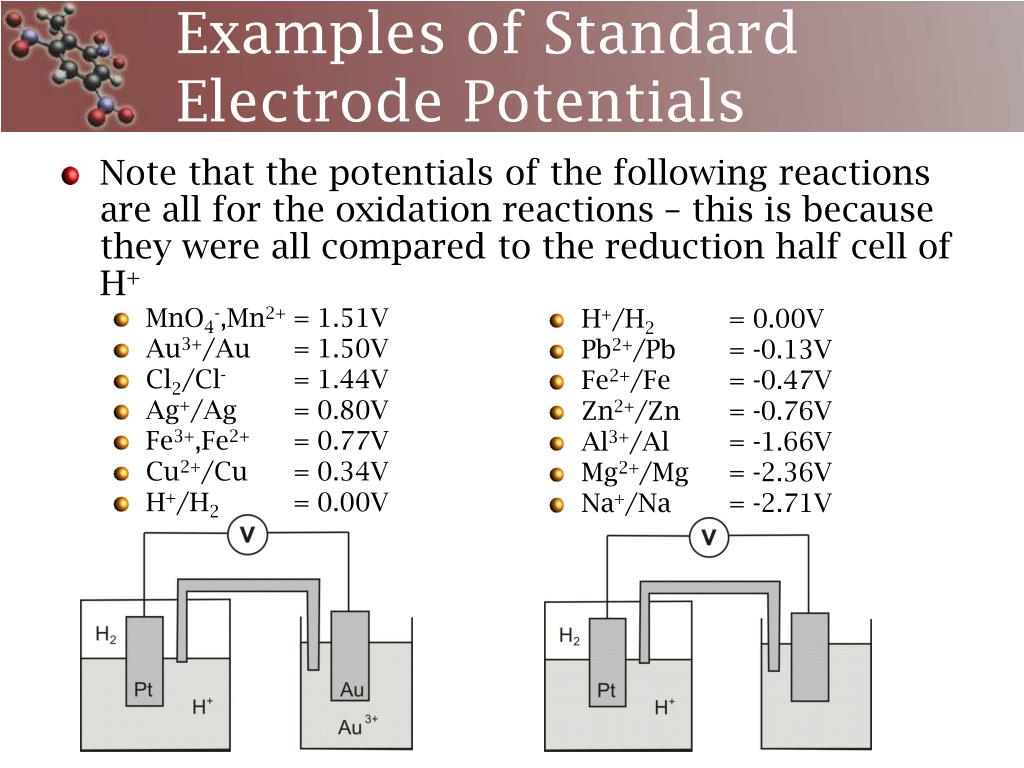
Standard Electrode Potential Voltage Equation . The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Determining a standard electrode potential using a standard hydrogen electrode.
from www.slideserve.com
Determining a standard electrode potential using a standard hydrogen electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero.
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint
Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. Determining a standard electrode potential using a standard hydrogen electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Voltage Equation The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. Determining a standard electrode potential using a standard hydrogen electrode. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. 372 rows the data below tabulates standard electrode potentials (e°),. Standard Electrode Potential Voltage Equation.
From www.nagwa.com
Question Video Calculating the Standard Reduction Potential of an Standard Electrode Potential Voltage Equation In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero.. Standard Electrode Potential Voltage Equation.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. Determining a standard electrode potential using a standard hydrogen electrode. The electrode used for comparison is called the standard hydrogen electrode (she), which. Standard Electrode Potential Voltage Equation.
From www.youtube.com
CLASS 12 JEE & NEET ELECTROCHEMISTRY NERNST EQUATION & ELECTRODE Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode.. Standard Electrode Potential Voltage Equation.
From www.youtube.com
Standard reduction potentials Redox reactions and electrochemistry Standard Electrode Potential Voltage Equation The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. Determining a standard electrode potential using a standard hydrogen electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The electrode used for comparison is. Standard Electrode Potential Voltage Equation.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Voltage Equation The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. Determining a standard electrode potential using a standard hydrogen electrode. The emf measured when a metal / metal ion electrode. Standard Electrode Potential Voltage Equation.
From brainly.in
16. Given standard electrode potentials Fe3+ + 3e Fe E = 0.036 Standard Electrode Potential Voltage Equation In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero.. Standard Electrode Potential Voltage Equation.
From f15.beauty
Standard Reduction Potential Table Standard Electrode Potential Voltage Equation In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode.. Standard Electrode Potential Voltage Equation.
From ablogwithadifference.com
Single Electrode Potential and Standard Electrode PotentialThe best 5 Standard Electrode Potential Voltage Equation The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that. Standard Electrode Potential Voltage Equation.
From www.youtube.com
Using Standard Electrode Potentials YouTube Standard Electrode Potential Voltage Equation In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen. Standard Electrode Potential Voltage Equation.
From www.slideserve.com
PPT Electrochemistry II PowerPoint Presentation, free download ID Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. Determining a standard electrode potential using a standard hydrogen electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\). Standard Electrode Potential Voltage Equation.
From www.vrogue.co
Electrochemistry Electrode Potential And Standard Ele vrogue.co Standard Electrode Potential Voltage Equation Determining a standard electrode potential using a standard hydrogen electrode. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) +. Standard Electrode Potential Voltage Equation.
From www.youtube.com
19.1 Standard electrode potential (HL) YouTube Standard Electrode Potential Voltage Equation The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to. Standard Electrode Potential Voltage Equation.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Voltage Equation The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under. Standard Electrode Potential Voltage Equation.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Electrode Potential Voltage Equation Determining a standard electrode potential using a standard hydrogen electrode. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The cell potential for a voltaic cell. Standard Electrode Potential Voltage Equation.
From dokumen.tips
(PDF) Standard Electrode Potentials James Mungall Electrode Standard Electrode Potential Voltage Equation In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. The emf measured. Standard Electrode Potential Voltage Equation.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Voltage Equation The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which. Standard Electrode Potential Voltage Equation.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Voltage Equation Determining a standard electrode potential using a standard hydrogen electrode. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The cell potential for a voltaic cell under standard conditions. Standard Electrode Potential Voltage Equation.
From testbook.com
Standard Electrode PotentialLearn Definition,Formula,Conditions Standard Electrode Potential Voltage Equation The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. Determining a standard electrode potential using a standard hydrogen electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. 372 rows the. Standard Electrode Potential Voltage Equation.
From pveducation.org
Standard Potential PVEducation Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. The emf measured. Standard Electrode Potential Voltage Equation.
From www.youtube.com
How to Calculate Standard Cell Potential and Voltage Part 1 Examples Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. Determining a standard electrode potential using a standard hydrogen electrode. The emf measured when a metal / metal ion electrode is coupled to. Standard Electrode Potential Voltage Equation.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Voltage Equation The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Determining a standard electrode potential using a standard hydrogen electrode. The emf measured when a metal / metal ion electrode is coupled to. Standard Electrode Potential Voltage Equation.
From 2012books.lardbucket.org
Standard Potentials Standard Electrode Potential Voltage Equation The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. Determining a standard electrode potential using a standard hydrogen electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) +. Standard Electrode Potential Voltage Equation.
From byjus.com
Standard electrode potentials are Fe2+/Fe; E° = 0.44 volts Fe3+/Fe2+; E Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode.. Standard Electrode Potential Voltage Equation.
From byjus.com
Standard electrode potentials are Fe2+/Fe; E° = 0.44 volts Fe3+/Fe2+; E Standard Electrode Potential Voltage Equation In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. Determining a standard electrode potential using a standard hydrogen electrode. The cell potential for a voltaic cell. Standard Electrode Potential Voltage Equation.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential Voltage Equation The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v,. Standard Electrode Potential Voltage Equation.
From www.chegg.com
Solved The standard electrode potential for the following Standard Electrode Potential Voltage Equation The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The cell potential for a voltaic cell under standard conditions can be calculated. Standard Electrode Potential Voltage Equation.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Voltage Equation The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero.. Standard Electrode Potential Voltage Equation.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to. Standard Electrode Potential Voltage Equation.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Electrode Potential Voltage Equation The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The electrode used. Standard Electrode Potential Voltage Equation.
From www.researchgate.net
Calculated standard electrode potentials for reactions in Equations Standard Electrode Potential Voltage Equation Determining a standard electrode potential using a standard hydrogen electrode. The electrode used for comparison is called the standard hydrogen electrode (she), which is deemed to have a potential of 0.00 v (zero. The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) +. Standard Electrode Potential Voltage Equation.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Voltage Equation The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. Determining a standard electrode potential using a standard hydrogen electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative. Standard Electrode Potential Voltage Equation.
From www.slideserve.com
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint Standard Electrode Potential Voltage Equation The cell potential for a voltaic cell under standard conditions can be calculated from the standard electrode. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. Standard Electrode Potential Voltage Equation.
From byjus.com
6. The standard electrode potential E^° I2/I , E^° Br /Br2 and E^° Fe Standard Electrode Potential Voltage Equation 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v,. Standard Electrode Potential Voltage Equation.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Voltage Equation The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. In this example, the standard reduction potential for \(\ce{zn^{2+}(aq) + 2e^{−} → zn(s)}\) is −0.76 v, which means that the. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard. Standard Electrode Potential Voltage Equation.