Acidity In Boiler Water Is Due To Presence Of . Feed water can also become. When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Alkalinity can occur in three different forms depending on the ph levels of the water. The effect exhibits rough pitted surfaces. Boiler water should be alkaline to save it from. Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing a reaction that forms iron oxide (rust). These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. Total alkalinity occurs from the sum of these three forms. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. the hard water is passed first through cation exchange column
from www.epa.gov
The effect exhibits rough pitted surfaces. the hard water is passed first through cation exchange column Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. Alkalinity can occur in three different forms depending on the ph levels of the water. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. Total alkalinity occurs from the sum of these three forms. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Boiler water should be alkaline to save it from. Feed water can also become.
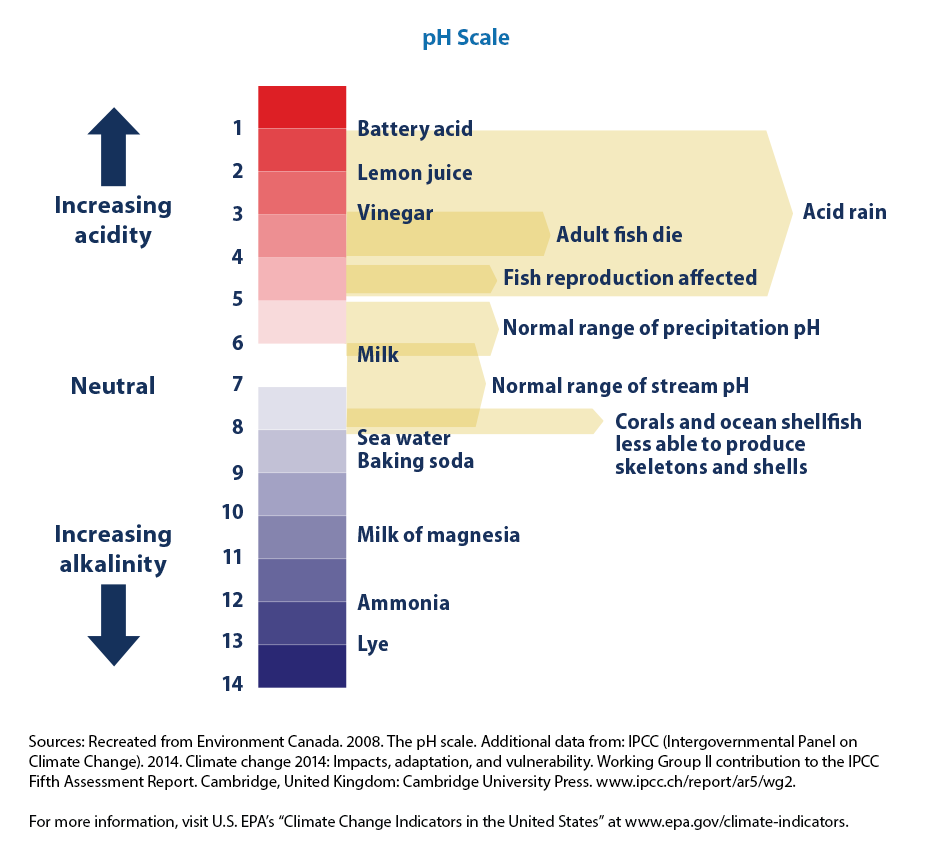
Climate Change Indicators Ocean Acidity US EPA
Acidity In Boiler Water Is Due To Presence Of In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. Feed water can also become. The effect exhibits rough pitted surfaces. Total alkalinity occurs from the sum of these three forms. Boiler water should be alkaline to save it from. Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing a reaction that forms iron oxide (rust). These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. the hard water is passed first through cation exchange column When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Alkalinity can occur in three different forms depending on the ph levels of the water.
From forum.heatinghelp.com
Testing PH level/Acidity on water boiler — Heating Help The Wall Acidity In Boiler Water Is Due To Presence Of the hard water is passed first through cation exchange column These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Boiler water should be alkaline to save it from. Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing a reaction that forms iron. Acidity In Boiler Water Is Due To Presence Of.
From 10feast.blogspot.com
What is Acidity? Symptoms, Causes and Treatment 10feast Acidity In Boiler Water Is Due To Presence Of These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. Total alkalinity occurs from the sum of these three forms. In boilers, the source of this corrosion could be dissolved gases in the boiler. Acidity In Boiler Water Is Due To Presence Of.
From possible.in
All you need to know about Symptoms and 11 Causes of Acidity! Acidity In Boiler Water Is Due To Presence Of When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Alkalinity can occur in three different forms depending on the ph levels of the water. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Total alkalinity occurs from the sum of these three forms. The effect exhibits. Acidity In Boiler Water Is Due To Presence Of.
From exoflhije.blob.core.windows.net
The Ph Value Of The Water Used In Boiler Is at Ernesto Barrera blog Acidity In Boiler Water Is Due To Presence Of Feed water can also become. The effect exhibits rough pitted surfaces. Total alkalinity occurs from the sum of these three forms. Boiler water should be alkaline to save it from. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Alkalinity can occur in three different forms depending on the ph levels of the. Acidity In Boiler Water Is Due To Presence Of.
From www.masterorganicchemistry.com
5 Key Factors That Influence Acidity In Organic Chemistry Acidity In Boiler Water Is Due To Presence Of Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. Boiler water should be alkaline to save it from. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). The effect exhibits rough pitted surfaces. the hard water is passed first through cation. Acidity In Boiler Water Is Due To Presence Of.
From marinerspointpro.com
Boiler Corrosion Causes,Effects and How to Prevent it Marinerspoint Pro Acidity In Boiler Water Is Due To Presence Of Alkalinity can occur in three different forms depending on the ph levels of the water. When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. The effect exhibits rough pitted surfaces. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will. Acidity In Boiler Water Is Due To Presence Of.
From www.researchgate.net
The water acidity ladder (as used in the "WTP to avoid further Acidity In Boiler Water Is Due To Presence Of Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing a reaction that forms iron oxide (rust). When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and. Acidity In Boiler Water Is Due To Presence Of.
From www.researchgate.net
Components of acidity in drainage water and pH for a drainage event in Acidity In Boiler Water Is Due To Presence Of Total alkalinity occurs from the sum of these three forms. Feed water can also become. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing. Acidity In Boiler Water Is Due To Presence Of.
From www.youtube.com
Determine Acidity of Drinking Water YouTube Acidity In Boiler Water Is Due To Presence Of Total alkalinity occurs from the sum of these three forms. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. Feed water can also become. the hard water is passed first through cation. Acidity In Boiler Water Is Due To Presence Of.
From www.differencebetween.com
Difference Between Acidity and Alkalinity of Water Compare the Acidity In Boiler Water Is Due To Presence Of Boiler water should be alkaline to save it from. Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing a reaction that forms iron oxide (rust). Feed water can also become. Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system.. Acidity In Boiler Water Is Due To Presence Of.
From pdfprof.com
alkalinity measurement Acidity In Boiler Water Is Due To Presence Of Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. The effect exhibits. Acidity In Boiler Water Is Due To Presence Of.
From studylib.net
Sludge and Scale formation in boilers In a boiler, water is Acidity In Boiler Water Is Due To Presence Of In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. Feed water can also become. The effect exhibits rough pitted surfaces. the hard water is passed first through cation exchange column Total. Acidity In Boiler Water Is Due To Presence Of.
From www.slideshare.net
Lab 4 alkalinity acidity and determination of alkalinity in water Acidity In Boiler Water Is Due To Presence Of The effect exhibits rough pitted surfaces. Feed water can also become. Alkalinity can occur in three different forms depending on the ph levels of the water. the hard water is passed first through cation exchange column Boiler water should be alkaline to save it from. Throughout a boiler system, the primary cause of corrosion is the presence of dissolved. Acidity In Boiler Water Is Due To Presence Of.
From exobjchpw.blob.core.windows.net
Acidity In Boiler Water Is Due To The Presence Of at Jay Moore blog Acidity In Boiler Water Is Due To Presence Of The effect exhibits rough pitted surfaces. the hard water is passed first through cation exchange column Boiler water should be alkaline to save it from. Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. In boilers, the source of this corrosion could be dissolved gases in the boiler. Acidity In Boiler Water Is Due To Presence Of.
From www.masterorganicchemistry.com
Acidity and Basicity of Alcohols Master Organic Chemistry Acidity In Boiler Water Is Due To Presence Of Total alkalinity occurs from the sum of these three forms. Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Boiler water should be alkaline to save it from. Feed water can also become.. Acidity In Boiler Water Is Due To Presence Of.
From dpuhospital.com
Causes of Acidity Comprehensive Overview DPU Hospital Acidity In Boiler Water Is Due To Presence Of Total alkalinity occurs from the sum of these three forms. Feed water can also become. Boiler water should be alkaline to save it from. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the. Acidity In Boiler Water Is Due To Presence Of.
From automaticheating.com.au
Guide to Managing the Condensate for Condensing Water Heaters. Acidity In Boiler Water Is Due To Presence Of These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). the hard water is passed first through cation exchange column Feed water can also become. The effect exhibits rough pitted surfaces. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which. Acidity In Boiler Water Is Due To Presence Of.
From www.youtube.com
Part1Boiler water chemistry Scale formation Turbidity, Conductivity Acidity In Boiler Water Is Due To Presence Of the hard water is passed first through cation exchange column Alkalinity can occur in three different forms depending on the ph levels of the water. The effect exhibits rough pitted surfaces. Total alkalinity occurs from the sum of these three forms. Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and. Acidity In Boiler Water Is Due To Presence Of.
From exobjchpw.blob.core.windows.net
Acidity In Boiler Water Is Due To The Presence Of at Jay Moore blog Acidity In Boiler Water Is Due To Presence Of The effect exhibits rough pitted surfaces. the hard water is passed first through cation exchange column When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing a reaction that forms iron oxide (rust). These. Acidity In Boiler Water Is Due To Presence Of.
From www.studocu.com
Unit 26 Acid and Base Chemistry Part II Unit 26. Factors that affect Acidity In Boiler Water Is Due To Presence Of Feed water can also become. Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. The effect exhibits rough pitted surfaces. Total alkalinity occurs from the sum of these three forms. Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing. Acidity In Boiler Water Is Due To Presence Of.
From lotusarise.com
Ocean Acidification Causes & its Effects UPSC UPSC Notes » LotusArise Acidity In Boiler Water Is Due To Presence Of Alkalinity can occur in three different forms depending on the ph levels of the water. Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Feed water can also become. Total alkalinity occurs from. Acidity In Boiler Water Is Due To Presence Of.
From www.studocu.com
Total Acidity OF Water Lab Practical notes TOTAL ACIDITY OF WATER Acidity In Boiler Water Is Due To Presence Of the hard water is passed first through cation exchange column Total alkalinity occurs from the sum of these three forms. Alkalinity can occur in three different forms depending on the ph levels of the water. When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Feed water can also become. In boilers, the. Acidity In Boiler Water Is Due To Presence Of.
From climatechange.ucdavis.edu
Ocean Acidification UC Davis Acidity In Boiler Water Is Due To Presence Of These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). the hard water is passed first through cation exchange column Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing a reaction that forms iron oxide (rust). In boilers, the source of this corrosion. Acidity In Boiler Water Is Due To Presence Of.
From exobjchpw.blob.core.windows.net
Acidity In Boiler Water Is Due To The Presence Of at Jay Moore blog Acidity In Boiler Water Is Due To Presence Of In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. Throughout a boiler system, the primary cause of corrosion is the presence of dissolved oxygen in the water, causing a reaction that forms. Acidity In Boiler Water Is Due To Presence Of.
From exobjchpw.blob.core.windows.net
Acidity In Boiler Water Is Due To The Presence Of at Jay Moore blog Acidity In Boiler Water Is Due To Presence Of the hard water is passed first through cation exchange column Feed water can also become. Boiler water should be alkaline to save it from. The effect exhibits rough pitted surfaces. When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. In boilers, the source of this corrosion could be dissolved gases in the. Acidity In Boiler Water Is Due To Presence Of.
From www.youtube.com
Acidity of Water Acidity Minerals Acidity & Total Acidity Acidity In Boiler Water Is Due To Presence Of Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. Boiler water should. Acidity In Boiler Water Is Due To Presence Of.
From www.kokilabenhospital.com
Watch out for these signs of acidity Acidity In Boiler Water Is Due To Presence Of When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Feed water can also become. Alkalinity can occur in three different forms depending on the ph levels of the water. The effect exhibits rough pitted surfaces. In boilers,. Acidity In Boiler Water Is Due To Presence Of.
From www.drdarrinlew.us
Acidity And Alkalinity Water Quality Dr. Darrin Lew Acidity In Boiler Water Is Due To Presence Of Boiler water should be alkaline to save it from. the hard water is passed first through cation exchange column Alkalinity can occur in three different forms depending on the ph levels of the water. The effect exhibits rough pitted surfaces. Feed water can also become. In boilers, the source of this corrosion could be dissolved gases in the boiler. Acidity In Boiler Water Is Due To Presence Of.
From www.dreamstime.com
Alkalinity PH Chart with Water Acidity from Bad To Ideal Outline Acidity In Boiler Water Is Due To Presence Of Total alkalinity occurs from the sum of these three forms. Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). The effect exhibits rough pitted surfaces. When the boiler water ph drops below about. Acidity In Boiler Water Is Due To Presence Of.
From www.chemwifi.com
Determination of Acidity in Water chemwifi Acidity In Boiler Water Is Due To Presence Of Feed water can also become. Boiler water should be alkaline to save it from. When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper. Acidity In Boiler Water Is Due To Presence Of.
From www.coursehero.com
[Solved] . Task 1 Determining total acidity in given water sample Acidity In Boiler Water Is Due To Presence Of Total alkalinity occurs from the sum of these three forms. Feed water can also become. When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. Boiler water should be alkaline to save it from. Alkalinity can occur in three different forms depending on the ph levels of the water. Throughout a boiler system, the. Acidity In Boiler Water Is Due To Presence Of.
From www.epa.gov
Climate Change Indicators Ocean Acidity US EPA Acidity In Boiler Water Is Due To Presence Of Total alkalinity occurs from the sum of these three forms. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Alkalinity can occur in three different forms depending on the ph levels of the water. the hard water is passed first through cation exchange column Low make up feed water ph can cause. Acidity In Boiler Water Is Due To Presence Of.
From exoflhije.blob.core.windows.net
The Ph Value Of The Water Used In Boiler Is at Ernesto Barrera blog Acidity In Boiler Water Is Due To Presence Of Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. In boilers, the source of this corrosion could be dissolved gases in the boiler water or the excessive use of hydrazine which will corrode copper and copper alloys, allowing copper to be carried back to the boiler. The effect exhibits. Acidity In Boiler Water Is Due To Presence Of.
From www.degruyter.com
Determination of alkalinity in the water sample a theoretical approach Acidity In Boiler Water Is Due To Presence Of These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Low make up feed water ph can cause serious acid attack on metal surfaces in the preboiler and boiler system. Boiler water should be alkaline to save it from. Total alkalinity occurs from the sum of these three forms. In boilers, the source of. Acidity In Boiler Water Is Due To Presence Of.
From www.differencebetween.com
Difference Between Acidity and Alkalinity of Water Compare the Acidity In Boiler Water Is Due To Presence Of The effect exhibits rough pitted surfaces. Alkalinity can occur in three different forms depending on the ph levels of the water. When the boiler water ph drops below about 8.5, a corrosion called acid attack can occur. These can come in the form of carbonate (co 3), bicarbonate (hco 3), or hydroxide (oh). Total alkalinity occurs from the sum of. Acidity In Boiler Water Is Due To Presence Of.