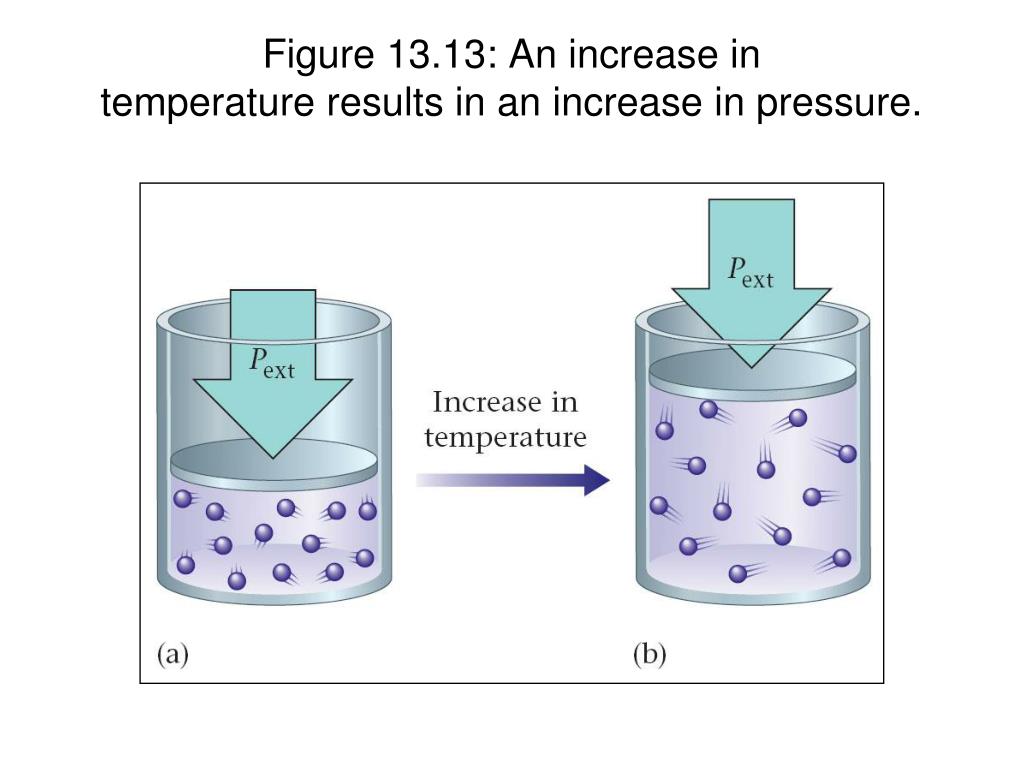
Does Pressure Increase When Temperature Decreases . To do that you can either add more particles into the same container, reduced the volume of the. Volume increases with increasing temperature or amount but decreases with increasing pressure. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing volume. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. Pressure increases when particles move faster. If we pull out the. In this scenario, the air is not confined. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; The following figure illustrates the.
from www.slideserve.com
Volume increases with increasing temperature or amount but decreases with increasing pressure. In reality, most compression take place by reducing volume. The following figure illustrates the. Pressure increases when particles move faster. If we pull out the. To do that you can either add more particles into the same container, reduced the volume of the. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; In this scenario, the air is not confined. At higher altitudes, the temperature is generally lower, and the air pressure is also lower.
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation
Does Pressure Increase When Temperature Decreases Pressure increases when particles move faster. Volume increases with increasing temperature or amount but decreases with increasing pressure. To do that you can either add more particles into the same container, reduced the volume of the. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; In reality, most compression take place by reducing volume. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The following figure illustrates the. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. Pressure increases when particles move faster. In this scenario, the air is not confined. If we pull out the.
From www.slideserve.com
PPT AP Chemistry Unit 2 PowerPoint Presentation, free download ID Does Pressure Increase When Temperature Decreases If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; In reality, most compression take place by reducing volume. The following figure illustrates the. Volume increases with increasing temperature or amount but decreases with increasing pressure. Pressure increases when particles move faster. If you. Does Pressure Increase When Temperature Decreases.
From owlcation.com
The Theories and Behavior of Gas Owlcation Does Pressure Increase When Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; Pressure increases when particles move faster. In this scenario, the air is. Does Pressure Increase When Temperature Decreases.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Does Pressure Increase When Temperature Decreases In this scenario, the air is not confined. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; Volume increases with increasing temperature or amount but decreases with increasing pressure. If we pull out the. The following figure illustrates the. If you had a. Does Pressure Increase When Temperature Decreases.
From gcsephysicsninja.com
11. Heating gas at a constant pressure Does Pressure Increase When Temperature Decreases If we pull out the. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; The following figure illustrates the. Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes, temperature would increase by. Does Pressure Increase When Temperature Decreases.
From www.toppr.com
The variation of pressure versus temperature of an ideal gas is shown Does Pressure Increase When Temperature Decreases If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; Pressure increases when particles move faster. Volume increases with increasing temperature or amount but decreases with increasing pressure. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. To. Does Pressure Increase When Temperature Decreases.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Does Pressure Increase When Temperature Decreases To do that you can either add more particles into the same container, reduced the volume of the. In reality, most compression take place by reducing volume. Pressure increases when particles move faster. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; Volume. Does Pressure Increase When Temperature Decreases.
From slidetodoc.com
The relationship between temperature and volume How Volume Does Pressure Increase When Temperature Decreases Volume increases with increasing temperature or amount but decreases with increasing pressure. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In reality, most compression take place by reducing volume. In this. Does Pressure Increase When Temperature Decreases.
From www.theweatherprediction.com
PRESSURE AND TEMPERATURE RELATIONSHIP Does Pressure Increase When Temperature Decreases In reality, most compression take place by reducing volume. To do that you can either add more particles into the same container, reduced the volume of the. Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. If we slowly push in. Does Pressure Increase When Temperature Decreases.
From general.chemistrysteps.com
Entropy and State Change Chemistry Steps Does Pressure Increase When Temperature Decreases In this scenario, the air is not confined. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. Volume increases with increasing temperature or amount but decreases with increasing pressure. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed. Does Pressure Increase When Temperature Decreases.
From docslib.org
Relationship Between Density, Pressure, and Temperature DocsLib Does Pressure Increase When Temperature Decreases The following figure illustrates the. To do that you can either add more particles into the same container, reduced the volume of the. If we pull out the. Volume increases with increasing temperature or amount but decreases with increasing pressure. Pressure increases when particles move faster. If you had a way to increase pressure with no volume change, then yes,. Does Pressure Increase When Temperature Decreases.
From www.majalahsains.com
Penjelasan Sains Mengenai Suhu dan Altitud MajalahSains Does Pressure Increase When Temperature Decreases If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; At higher altitudes, the temperature is generally lower, and the air pressure is also lower. To do that you can either add more particles into the same container, reduced the volume of the. The. Does Pressure Increase When Temperature Decreases.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Does Pressure Increase When Temperature Decreases The following figure illustrates the. In reality, most compression take place by reducing volume. Volume increases with increasing temperature or amount but decreases with increasing pressure. In this scenario, the air is not confined. To do that you can either add more particles into the same container, reduced the volume of the. If we slowly push in the plunger while. Does Pressure Increase When Temperature Decreases.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Does Pressure Increase When Temperature Decreases To do that you can either add more particles into the same container, reduced the volume of the. Volume increases with increasing temperature or amount but decreases with increasing pressure. If we pull out the. In this scenario, the air is not confined. Pressure increases when particles move faster. In reality, most compression take place by reducing volume. If we. Does Pressure Increase When Temperature Decreases.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Does Pressure Increase When Temperature Decreases The following figure illustrates the. Volume increases with increasing temperature or amount but decreases with increasing pressure. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; To do that you can either add more particles into the same container, reduced the volume of. Does Pressure Increase When Temperature Decreases.
From courses.lumenlearning.com
Phase Changes Physics Does Pressure Increase When Temperature Decreases If we pull out the. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The following figure illustrates the. In this scenario, the air is not confined. Pressure increases when particles move faster. At higher altitudes, the temperature is generally lower, and the air pressure is also. Does Pressure Increase When Temperature Decreases.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Does Pressure Increase When Temperature Decreases At higher altitudes, the temperature is generally lower, and the air pressure is also lower. In this scenario, the air is not confined. If we pull out the. In reality, most compression take place by reducing volume. Pressure increases when particles move faster. The following figure illustrates the. To do that you can either add more particles into the same. Does Pressure Increase When Temperature Decreases.
From www.acurite.com
Atmospheric Pressure AcuRite Does Pressure Increase When Temperature Decreases Pressure increases when particles move faster. The following figure illustrates the. If we pull out the. In this scenario, the air is not confined. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. Does Pressure Increase When Temperature Decreases.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Pressure Increase When Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The following figure illustrates the. If we pull out the. In reality, most compression take place by reducing volume. Volume increases with increasing temperature or amount but decreases with increasing pressure. In this scenario, the air is not. Does Pressure Increase When Temperature Decreases.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Does Pressure Increase When Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. The following figure illustrates the. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If we pull out the. Pressure increases when particles move faster. If we slowly push in the plunger. Does Pressure Increase When Temperature Decreases.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Does Pressure Increase When Temperature Decreases If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; The following figure illustrates the. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If we pull out the. In this scenario, the air is not confined. If. Does Pressure Increase When Temperature Decreases.
From www.slideshare.net
10.2 First law of Thermodynamics and PV graphs Does Pressure Increase When Temperature Decreases In reality, most compression take place by reducing volume. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; If we pull out the. In this scenario, the air is not confined. Volume increases with increasing temperature or amount but decreases with increasing pressure.. Does Pressure Increase When Temperature Decreases.
From www.slideshare.net
Air pressure Does Pressure Increase When Temperature Decreases At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. If we pull out the. Pressure increases when particles move faster. If we slowly push in the plunger while keeping temperature constant, the. Does Pressure Increase When Temperature Decreases.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Does Pressure Increase When Temperature Decreases In reality, most compression take place by reducing volume. Volume increases with increasing temperature or amount but decreases with increasing pressure. The following figure illustrates the. Pressure increases when particles move faster. To do that you can either add more particles into the same container, reduced the volume of the. At higher altitudes, the temperature is generally lower, and the. Does Pressure Increase When Temperature Decreases.
From med.libretexts.org
2.3 Gaseous Exchange Mechanism Medicine LibreTexts Does Pressure Increase When Temperature Decreases To do that you can either add more particles into the same container, reduced the volume of the. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. In reality, most compression take place by reducing volume. Pressure increases when particles move faster. In this scenario, the air is not confined. Volume increases with increasing. Does Pressure Increase When Temperature Decreases.
From www.youtube.com
Why temperature decreases with altitude?why_temperature_decreases_with Does Pressure Increase When Temperature Decreases In reality, most compression take place by reducing volume. To do that you can either add more particles into the same container, reduced the volume of the. In this scenario, the air is not confined. Volume increases with increasing temperature or amount but decreases with increasing pressure. If we pull out the. Pressure increases when particles move faster. If you. Does Pressure Increase When Temperature Decreases.
From www.shalom-education.com
Volume and Pressure in Gases GCSE Physics Revision Does Pressure Increase When Temperature Decreases In this scenario, the air is not confined. Pressure increases when particles move faster. To do that you can either add more particles into the same container, reduced the volume of the. Volume increases with increasing temperature or amount but decreases with increasing pressure. If we pull out the. At higher altitudes, the temperature is generally lower, and the air. Does Pressure Increase When Temperature Decreases.
From www.slideserve.com
PPT Unit 2, lesson 2 Temperature PowerPoint Presentation, free Does Pressure Increase When Temperature Decreases In reality, most compression take place by reducing volume. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; Volume increases with increasing temperature or amount but decreases with increasing pressure. At higher altitudes, the temperature is generally lower, and the air pressure is. Does Pressure Increase When Temperature Decreases.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Does Pressure Increase When Temperature Decreases In this scenario, the air is not confined. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; The following figure illustrates the. To do that you can either add more particles into the same container, reduced the volume of the. Volume increases with. Does Pressure Increase When Temperature Decreases.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Does Pressure Increase When Temperature Decreases In this scenario, the air is not confined. In reality, most compression take place by reducing volume. Pressure increases when particles move faster. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. The following figure illustrates the. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is. Does Pressure Increase When Temperature Decreases.
From www.w3schools.blog
Factors affecting the rate of a reaction Temperature W3schools Does Pressure Increase When Temperature Decreases If we pull out the. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. Volume increases with increasing temperature or amount but decreases with increasing pressure. To do that you can either add more particles into the same container, reduced the volume of the. Pressure increases when particles move faster. In reality, most compression. Does Pressure Increase When Temperature Decreases.
From www.youtube.com
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry YouTube Does Pressure Increase When Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In this scenario, the air is not confined. Pressure increases when particles move faster. Volume increases with increasing temperature or amount but decreases with increasing pressure. At higher altitudes, the temperature is generally lower, and the air pressure. Does Pressure Increase When Temperature Decreases.
From slideplayer.com
If temperature decreases, what happens to pressure? ppt download Does Pressure Increase When Temperature Decreases The following figure illustrates the. If we pull out the. Volume increases with increasing temperature or amount but decreases with increasing pressure. At higher altitudes, the temperature is generally lower, and the air pressure is also lower. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and. Does Pressure Increase When Temperature Decreases.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Does Pressure Increase When Temperature Decreases Pressure increases when particles move faster. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into a smaller volume and its pressure increases; At higher altitudes, the temperature is generally lower, and the air pressure is also lower. In this scenario, the air is not confined. In reality, most compression take. Does Pressure Increase When Temperature Decreases.
From www.savemyexams.co.uk
Gas Law Relationships (1.2.5) IB DP Chemistry SL Revision Notes 2016 Does Pressure Increase When Temperature Decreases If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. In this scenario, the air is not confined. Pressure increases when particles move faster. If we pull out the. If we slowly push in the plunger while keeping temperature constant, the gas in the syringe is compressed into. Does Pressure Increase When Temperature Decreases.
From www.nagwa.com
Question Video Identifying the Relationship between the Pressure and Does Pressure Increase When Temperature Decreases The following figure illustrates the. In reality, most compression take place by reducing volume. If we pull out the. In this scenario, the air is not confined. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas law. At higher altitudes, the temperature is generally lower, and the air. Does Pressure Increase When Temperature Decreases.