Bromide Ion Dissolved In Water . The solution is acidified by adding dilute nitric acid. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Define a solution and describe the parts of a solution. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. Describe how an aqueous solution is formed from both ionic compounds. Most bromides are soluble in water. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. See the study guide on the. Silver nitrate + dilute nitric acid.) the nitric acid. If you start from a solid, it must first be dissolved in pure water.
from www.numerade.com
Most bromides are soluble in water. See the study guide on the. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Silver nitrate + dilute nitric acid.) the nitric acid. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. The solution is acidified by adding dilute nitric acid. Describe how an aqueous solution is formed from both ionic compounds. Define a solution and describe the parts of a solution. If you start from a solid, it must first be dissolved in pure water.
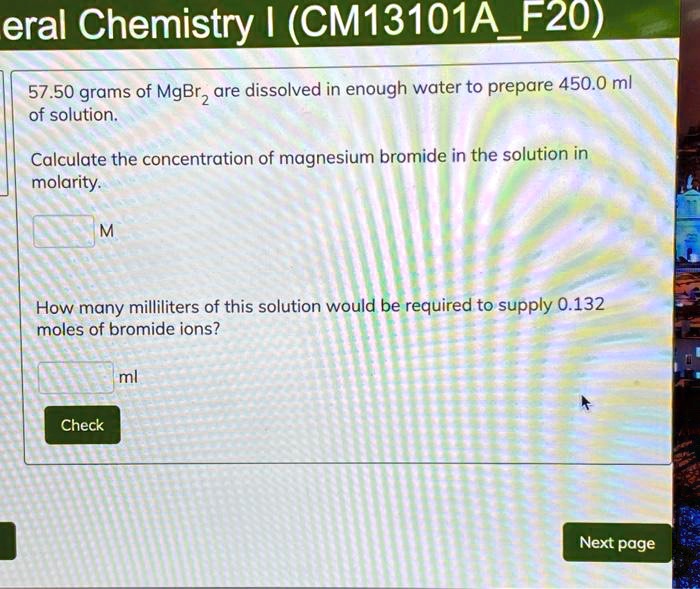
SOLVED General Chemistry (CM13101A F20) 57.50 grams of MgBr2 are
Bromide Ion Dissolved In Water Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Silver nitrate + dilute nitric acid.) the nitric acid. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Define a solution and describe the parts of a solution. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. Most bromides are soluble in water. If you start from a solid, it must first be dissolved in pure water. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Describe how an aqueous solution is formed from both ionic compounds. The solution is acidified by adding dilute nitric acid. See the study guide on the.
From www.numerade.com
The electrolysis of an aqueous solution of magnesium bromide (MgBr2 Bromide Ion Dissolved In Water Most bromides are soluble in water. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. Describe how an aqueous solution is formed from both ionic compounds. If you start from a solid, it must first be dissolved in pure water. The solution is acidified by adding dilute nitric acid. When some substances are dissolved. Bromide Ion Dissolved In Water.
From www.chemistryworld.com
Potassium bromide Podcast Chemistry World Bromide Ion Dissolved In Water See the study guide on the. The solution is acidified by adding dilute nitric acid. If you start from a solid, it must first be dissolved in pure water. Silver nitrate + dilute nitric acid.) the nitric acid. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Nitrates are. Bromide Ion Dissolved In Water.
From www.fishersci.com
Bromoacetyl bromide, 98, Thermo Scientific Chemicals, Quantity 25 g Bromide Ion Dissolved In Water Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Define a solution and describe the parts of a solution. Describe how an aqueous solution is formed from both ionic compounds. If. Bromide Ion Dissolved In Water.
From www.rpicorp.com
E718005.0 Ethidium Bromide, Powder, 5 Grams Bromide Ion Dissolved In Water See the study guide on the. Silver nitrate + dilute nitric acid.) the nitric acid. The solution is acidified by adding dilute nitric acid. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. If you start from a solid, it must first be dissolved in pure water. Review solubility rules for common ionic compounds. Bromide Ion Dissolved In Water.
From www.chegg.com
Solved A 0.5019 g sample of a pure soluble bromide compound Bromide Ion Dissolved In Water Silver nitrate + dilute nitric acid.) the nitric acid. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Suggest an explanation for a reason why it may be difficult to distinguish. Bromide Ion Dissolved In Water.
From www.dreamstime.com
Potassium Bromide Chemical Formula on Waterdrop Background Stock Bromide Ion Dissolved In Water If you start from a solid, it must first be dissolved in pure water. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. The solution is acidified by adding dilute nitric acid. See the study guide on the. Define a solution and describe the parts of. Bromide Ion Dissolved In Water.
From www.numerade.com
SOLVED General Chemistry (CM13101A F20) 57.50 grams of MgBr2 are Bromide Ion Dissolved In Water If you start from a solid, it must first be dissolved in pure water. Define a solution and describe the parts of a solution. The solution is acidified by adding dilute nitric acid. Most bromides are soluble in water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. When some substances. Bromide Ion Dissolved In Water.
From klanamqvi.blob.core.windows.net
Why Are Ionic Compounds Easily Soluble In Water at Tom Ungar blog Bromide Ion Dissolved In Water Define a solution and describe the parts of a solution. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. If you start from a solid, it must first be dissolved. Bromide Ion Dissolved In Water.
From www.coursehero.com
[Solved] A 1.362 g sample of calcium bromide is dissolved in enough Bromide Ion Dissolved In Water Describe how an aqueous solution is formed from both ionic compounds. The solution is acidified by adding dilute nitric acid. Most bromides are soluble in water. See the study guide on the. If you start from a solid, it must first be dissolved in pure water. Suggest an explanation for a reason why it may be difficult to distinguish between. Bromide Ion Dissolved In Water.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Bromide Ion Dissolved In Water The solution is acidified by adding dilute nitric acid. See the study guide on the. If you start from a solid, it must first be dissolved in pure water. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Define a solution and describe the parts of a solution. Silver nitrate +. Bromide Ion Dissolved In Water.
From www.numerade.com
SOLVED Calculate the number of bromide ions when 4.23 g of magnesium Bromide Ion Dissolved In Water Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. The solution is acidified by adding dilute nitric acid. If you start from a solid, it must first be dissolved in pure water. Most bromides are soluble in water. Describe how an aqueous solution is formed from both ionic compounds. See the. Bromide Ion Dissolved In Water.
From www.chegg.com
A small amount of sodium bromide (NaBr) is dissolved Bromide Ion Dissolved In Water Most bromides are soluble in water. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Describe how an aqueous solution is formed from both ionic compounds. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. When some substances are dissolved. Bromide Ion Dissolved In Water.
From www.numerade.com
SOLVED The Solubility Product Constant for chromium(I) bromide is > 1 Bromide Ion Dissolved In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Most bromides are soluble in water. Define a solution and describe the parts of a solution. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. If you. Bromide Ion Dissolved In Water.
From www.numerade.com
Major species present dissolved in water when Formula compound Bromide Ion Dissolved In Water Describe how an aqueous solution is formed from both ionic compounds. Silver nitrate + dilute nitric acid.) the nitric acid. See the study guide on the. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Most bromides are soluble in water. When some substances are dissolved. Bromide Ion Dissolved In Water.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromide Ion Dissolved In Water Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. The solution is acidified by adding dilute nitric acid. Describe how an aqueous solution is formed from both ionic compounds. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Suggest an explanation for a reason. Bromide Ion Dissolved In Water.
From www.numerade.com
A sample of 0.3220 g of an ionic compound containing the bromide ion Bromide Ion Dissolved In Water Describe how an aqueous solution is formed from both ionic compounds. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Define a solution and describe the parts of a solution. Most bromides are soluble in water. See the study guide on the. The solution is acidified. Bromide Ion Dissolved In Water.
From www.numerade.com
SOLVED Determine the normality of bromide ion when 0.786 g of calcium Bromide Ion Dissolved In Water Most bromides are soluble in water. Describe how an aqueous solution is formed from both ionic compounds. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Define a solution and describe the parts of a solution. The solution is acidified by adding dilute nitric acid. When some substances are dissolved in. Bromide Ion Dissolved In Water.
From chemcraft.su
Lead(II) bromide, 99.5 pure chemcraft.su Bromide Ion Dissolved In Water See the study guide on the. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. Define a solution and describe the parts of a solution. Describe how an aqueous solution is formed from both ionic compounds. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields. Bromide Ion Dissolved In Water.
From www.chegg.com
Solved A small amount of sodium bromide (NaBr) is dissolved Bromide Ion Dissolved In Water Define a solution and describe the parts of a solution. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. See the study guide on the. If you start from a solid, it must first be dissolved in pure water. Silver nitrate + dilute nitric acid.) the nitric acid. Ionic compounds have high melting and. Bromide Ion Dissolved In Water.
From www.sciencephoto.com
Bromide ion in water molecules Stock Image A504/0046 Science Bromide Ion Dissolved In Water Most bromides are soluble in water. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. If you start from a solid, it must first be dissolved in pure water. Silver nitrate + dilute nitric acid.) the nitric acid. Define a solution and describe the parts of. Bromide Ion Dissolved In Water.
From www.numerade.com
SOLVED A 0.8838 g sample of an ionic compound containing bromide ions Bromide Ion Dissolved In Water If you start from a solid, it must first be dissolved in pure water. The solution is acidified by adding dilute nitric acid. Define a solution and describe the parts of a solution. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Nitrates are soluble in water with no exceptions, so. Bromide Ion Dissolved In Water.
From www.youtube.com
Equation for NaBr + H2O (Sodium bromide + Water) YouTube Bromide Ion Dissolved In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide. Bromide Ion Dissolved In Water.
From www.chegg.com
Solved A small amount of sodium bromide (NaBr) is dissolved Bromide Ion Dissolved In Water Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. Define a solution and describe the parts of a solution. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. If you start from a solid, it must first be dissolved in pure water. Most bromides are. Bromide Ion Dissolved In Water.
From www.thistlescientific.co.uk
Ethidium Bromide solution 10mg/ml Thistle Scientific Bromide Ion Dissolved In Water See the study guide on the. If you start from a solid, it must first be dissolved in pure water. Most bromides are soluble in water. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Suggest an explanation for a reason why it may be difficult to distinguish between. Bromide Ion Dissolved In Water.
From www.numerade.com
SOLVED 1.A sample of 0.3220 g of an ionic compound containing the Bromide Ion Dissolved In Water The solution is acidified by adding dilute nitric acid. See the study guide on the. Silver nitrate + dilute nitric acid.) the nitric acid. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Most bromides are soluble in water. Describe how an aqueous solution is formed from both ionic compounds.. Bromide Ion Dissolved In Water.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Bromide Ion Dissolved In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate,. Bromide Ion Dissolved In Water.
From www.numerade.com
A small amount of sodium bromide (NaBr) dissolved in a large amount of Bromide Ion Dissolved In Water See the study guide on the. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. Bromide Ion Dissolved In Water.
From www.youtube.com
Bromide ions with chlorine water YouTube Bromide Ion Dissolved In Water Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Silver nitrate + dilute nitric acid.) the nitric acid. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Suggest an explanation for a reason why it may be difficult to distinguish. Bromide Ion Dissolved In Water.
From www.dreamstime.com
3D Image of Vinyl Bromide Skeletal Formula Stock Illustration Bromide Ion Dissolved In Water Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. The solution is acidified by adding dilute nitric acid. Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Most bromides are soluble in water. Review solubility rules for. Bromide Ion Dissolved In Water.
From slideplayer.com
Essential Organic Chemistry ppt download Bromide Ion Dissolved In Water Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Define a solution and describe the parts of a solution. Silver nitrate + dilute nitric acid.) the nitric. Bromide Ion Dissolved In Water.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Bromide Ion Dissolved In Water When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in. Most bromides are soluble in water. Silver nitrate + dilute nitric acid.) the nitric acid. Describe how an aqueous solution is formed from both ionic compounds. Ionic compounds have high melting and boiling points, so they are in the solid. Bromide Ion Dissolved In Water.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Bromide Ion Dissolved In Water If you start from a solid, it must first be dissolved in pure water. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. The solution is acidified. Bromide Ion Dissolved In Water.
From sielc.com
Vinyl bromide SIELC Technologies Bromide Ion Dissolved In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. Describe how an aqueous solution is formed from both ionic compounds. Most bromides are soluble in water. Nitrates are soluble in water with no exceptions, so zn (no 3) 2 is soluble. When some substances are dissolved in water, they undergo. Bromide Ion Dissolved In Water.
From www.chegg.com
Solved 13) (4 points) Sodium bromide, NaBr, is dissolved in Bromide Ion Dissolved In Water Ionic compounds have high melting and boiling points, so they are in the solid state at room temperature. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. See the study guide on the. Silver nitrate + dilute nitric acid.) the nitric acid. Suggest an explanation for a reason why it. Bromide Ion Dissolved In Water.
From www.eurekalert.org
Fig. 1 [IMAGE] EurekAlert! Science News Releases Bromide Ion Dissolved In Water Define a solution and describe the parts of a solution. Silver nitrate + dilute nitric acid.) the nitric acid. See the study guide on the. The solution is acidified by adding dilute nitric acid. Suggest an explanation for a reason why it may be difficult to distinguish between very dilute solutions of chloride ions, bromide and iodide. Nitrates are soluble. Bromide Ion Dissolved In Water.