Copper Oxide Formula Balanced . 1 atom in reagents and 2 atoms in products. If the coefficient 2 is added in front, that makes 2 water molecules; 2cu + o 2 → 2cuo. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The copper atom is coordinated by 4 oxygen atoms. Original molecule h2o h 2 o: Here is the balanced symbol equation. If they weigh the reactants and products. For example, copper and oxygen react together to make. Copper + dioxygen = copper (ii) oxide. Balanced symbol equations show what happens to the different atoms in reactions. But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. 1 atom in reagents and 1 atom in products. In order to balance o. The balanced equation will appear.
from kristophernpetton.blogspot.com
For example, copper and oxygen react together to make. 2cu + o 2 → 2cuo. 1 atom in reagents and 2 atoms in products. If they weigh the reactants and products. Copper + dioxygen = copper (ii) oxide. In order to balance o. Copper (ii) oxide belongs to the monoclinic crystal system. The balanced equation will appear. You can see that now there are two copper atoms and two oxygen atoms on each. Balanced symbol equations show what happens to the different atoms in reactions.
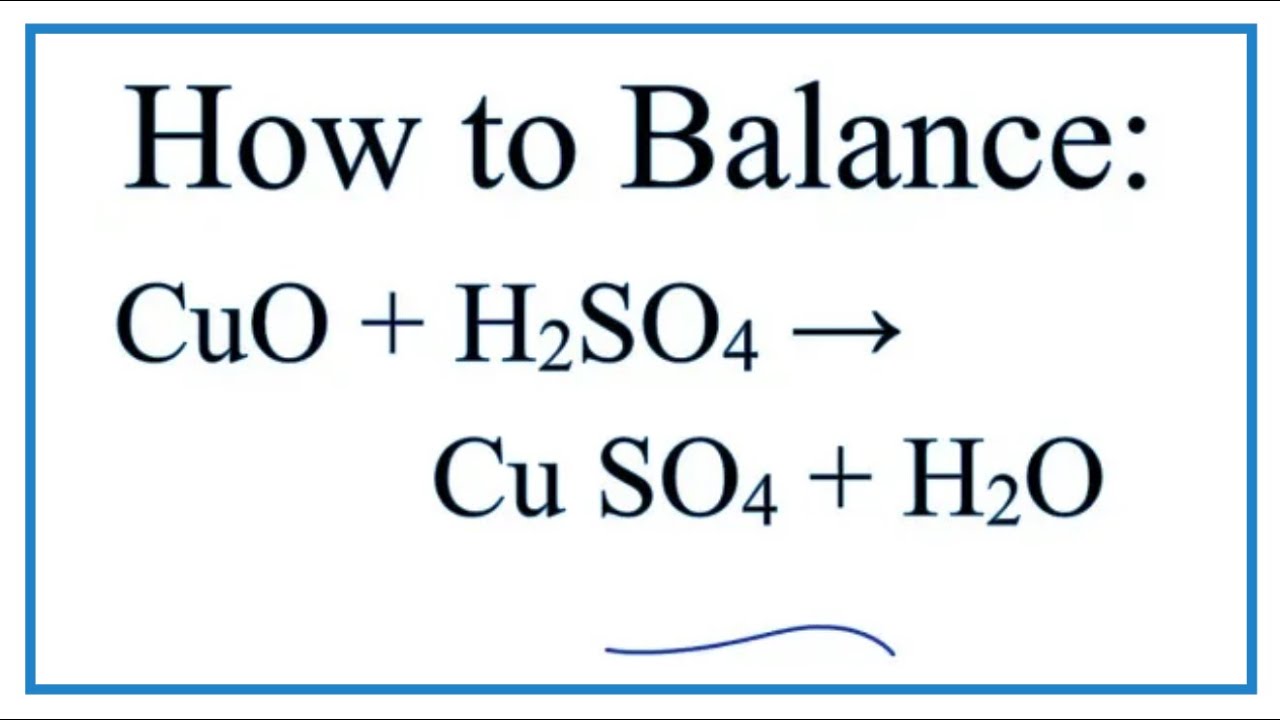
Copper Oxide and Sulfuric Acid Formula KristophernPetton
Copper Oxide Formula Balanced Original molecule h2o h 2 o: Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole. For example, copper and oxygen react together to make. The copper atom is coordinated by 4 oxygen atoms. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. You can see that now there are two copper atoms and two oxygen atoms on each. Here is the balanced symbol equation. Original molecule h2o h 2 o: 1 atom in reagents and 1 atom in products. If they weigh the reactants and products. But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. 2cu + o 2 → 2cuo. 1 atom in reagents and 2 atoms in products. Copper + dioxygen = copper (ii) oxide. Copper (ii) oxide belongs to the monoclinic crystal system.
From brainly.in
balanced equation for copper powder is heated strongly in air and Copper Oxide Formula Balanced The balanced equation will appear. Balanced symbol equations show what happens to the different atoms in reactions. 1 atom in reagents and 2 atoms in products. You can see that now there are two copper atoms and two oxygen atoms on each. Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole.. Copper Oxide Formula Balanced.
From www.chemtube3d.com
Copper (I) Oxide Cu2O Copper Oxide Formula Balanced The copper atom is coordinated by 4 oxygen atoms. The balanced equation will appear. Original molecule h2o h 2 o: Copper + dioxygen = copper (ii) oxide. You can see that now there are two copper atoms and two oxygen atoms on each. 2cu + o 2 → 2cuo. For example, copper and oxygen react together to make. If the. Copper Oxide Formula Balanced.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation ID5482147 Copper Oxide Formula Balanced 2cu + o 2 → 2cuo. For example, copper and oxygen react together to make. If they weigh the reactants and products. Copper (ii) oxide belongs to the monoclinic crystal system. 1 atom in reagents and 2 atoms in products. 1 atom in reagents and 1 atom in products. Cu + o2 = cuo is a synthesis reaction where two. Copper Oxide Formula Balanced.
From resource.studiaacademy.com
Chemical Formulae, Equations and Calculations Studia Academy Resources Copper Oxide Formula Balanced To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. The balanced equation will appear. Here is the balanced symbol equation. If they weigh the reactants and products. If the coefficient. Copper Oxide Formula Balanced.
From loeqyugeg.blob.core.windows.net
Copper(Ii) Oxide And Carbon Equation at John Wyllie blog Copper Oxide Formula Balanced But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. 1 atom in reagents and 1 atom in products. For example, copper and oxygen react together to make. Copper + dioxygen = copper (ii) oxide. 2cu + o 2 → 2cuo. In this experiment, students heat copper(ii) oxide in a glass tube while. Copper Oxide Formula Balanced.
From link.springer.com
Copper(II) oxide nanocatalyst preparation and characterization green Copper Oxide Formula Balanced You can see that now there are two copper atoms and two oxygen atoms on each. Balanced symbol equations show what happens to the different atoms in reactions. 1 atom in reagents and 1 atom in products. Original molecule h2o h 2 o: 1 atom in reagents and 2 atoms in products. 2cu + o 2 → 2cuo. But if. Copper Oxide Formula Balanced.
From www.slideserve.com
PPT Copper Functioning as an Oxygen Carrier in Chemical Looping Copper Oxide Formula Balanced In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. The copper atom is coordinated by 4 oxygen atoms. 1 atom in reagents and 2 atoms in products. The balanced equation will appear. Copper + dioxygen = copper (ii) oxide. 2cu + o 2 → 2cuo. Cu +. Copper Oxide Formula Balanced.
From byjus.com
If 1gram of copper on oxidation gives 1.252grams of copper oxide. Find Copper Oxide Formula Balanced Copper (ii) oxide belongs to the monoclinic crystal system. 1 atom in reagents and 2 atoms in products. In order to balance o. The balanced equation will appear. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Here is the balanced symbol equation. Copper + dioxygen =. Copper Oxide Formula Balanced.
From www.youtube.com
CuO+HCl=CuCl2+H2O Balanced EquationCopper oxide+Hydrochloric acid Copper Oxide Formula Balanced Balanced symbol equations show what happens to the different atoms in reactions. In order to balance o. But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. Copper + dioxygen = copper (ii) oxide. If they weigh the reactants and products. 2cu + o 2 → 2cuo. The balanced equation will appear. 1. Copper Oxide Formula Balanced.
From kristophernpetton.blogspot.com
Copper Oxide and Sulfuric Acid Formula KristophernPetton Copper Oxide Formula Balanced Original molecule h2o h 2 o: 1 atom in reagents and 2 atoms in products. In order to balance o. Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole. Balanced symbol equations show what happens to the different atoms in reactions. Copper + dioxygen = copper (ii) oxide. Copper (ii) oxide. Copper Oxide Formula Balanced.
From www.youtube.com
Lab Lecture Empirical Formula of Copper Oxide YouTube Copper Oxide Formula Balanced 1 atom in reagents and 1 atom in products. But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. You can see that now there are two copper atoms and two oxygen atoms on each. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Copper. Copper Oxide Formula Balanced.
From www.youtube.com
Chemical Calculations 1.16 Eqs & Calc/Masses to Balanced Equations Copper Oxide Formula Balanced Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole. In order to balance o. You can see that now there are two copper atoms and two oxygen atoms on each. If the coefficient 2 is added in front, that makes 2 water molecules; Original molecule h2o h 2 o: 1 atom. Copper Oxide Formula Balanced.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper Oxide Formula Balanced In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole. Original molecule h2o h 2 o: But if the subscript 2 is added to make h2o2 h 2 o 2,. Copper Oxide Formula Balanced.
From studylib.net
Copper Oxide Lab Copper Oxide Formula Balanced 1 atom in reagents and 1 atom in products. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. 2cu + o 2 → 2cuo. For example, copper and oxygen react together to make. If they weigh the reactants and products. Here is the balanced symbol equation. But if the subscript 2 is. Copper Oxide Formula Balanced.
From www.numerade.com
SOLVEDWhen ammonia gas is reacted with copper(II) oxide, nitrogen gas Copper Oxide Formula Balanced For example, copper and oxygen react together to make. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. You can see that now there are two copper atoms and two oxygen atoms on each. Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole.. Copper Oxide Formula Balanced.
From www.toppr.com
Write balanced chemical equations for the following word equation Copper Oxide Formula Balanced If they weigh the reactants and products. Copper + dioxygen = copper (ii) oxide. 1 atom in reagents and 1 atom in products. The copper atom is coordinated by 4 oxygen atoms. Here is the balanced symbol equation. If the coefficient 2 is added in front, that makes 2 water molecules; Balanced symbol equations show what happens to the different. Copper Oxide Formula Balanced.
From www.slideshare.net
Chapter 4 Copper Oxide Formula Balanced In order to balance o. But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. 1 atom in reagents and 2 atoms in products. Copper (ii) oxide belongs to the monoclinic crystal system. 2cu + o 2 → 2cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane. Copper Oxide Formula Balanced.
From www.toppr.com
Write balanced chemical equations for the following word equation Copper Oxide Formula Balanced 1 atom in reagents and 2 atoms in products. The balanced equation will appear. Balanced symbol equations show what happens to the different atoms in reactions. If the coefficient 2 is added in front, that makes 2 water molecules; Copper + dioxygen = copper (ii) oxide. If they weigh the reactants and products. But if the subscript 2 is added. Copper Oxide Formula Balanced.
From www.toppr.com
Write a balanced chemical equation for each of the followingReaction Copper Oxide Formula Balanced 2cu + o 2 → 2cuo. Original molecule h2o h 2 o: If the coefficient 2 is added in front, that makes 2 water molecules; For example, copper and oxygen react together to make. The copper atom is coordinated by 4 oxygen atoms. The balanced equation will appear. Copper (ii) oxide belongs to the monoclinic crystal system. 1 atom in. Copper Oxide Formula Balanced.
From www.numerade.com
SOLVEDWrite a balanced chemical equation for each reaction. a. Solid Copper Oxide Formula Balanced To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. For example, copper and oxygen react together to make. Copper (ii) oxide belongs to the monoclinic crystal system. 1 atom in reagents and 1 atom in. Copper Oxide Formula Balanced.
From www.numerade.com
SOLVED 12.0 g of copper is concerted to copper (II) oxide by heating Copper Oxide Formula Balanced Balanced symbol equations show what happens to the different atoms in reactions. Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole. 2cu + o 2 → 2cuo. Copper (ii) oxide belongs to the monoclinic crystal system. Original molecule h2o h 2 o: 1 atom in reagents and 1 atom in products.. Copper Oxide Formula Balanced.
From www.youtube.com
How to Write the Formula for Copper (I) oxide YouTube Copper Oxide Formula Balanced Balanced symbol equations show what happens to the different atoms in reactions. You can see that now there are two copper atoms and two oxygen atoms on each. The copper atom is coordinated by 4 oxygen atoms. In order to balance o. 2cu + o 2 → 2cuo. Original molecule h2o h 2 o: For example, copper and oxygen react. Copper Oxide Formula Balanced.
From brainly.in
When Cu(OH)2is heated, copper(II) oxide and water are formed. How would Copper Oxide Formula Balanced For example, copper and oxygen react together to make. Here is the balanced symbol equation. Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole. Copper + dioxygen = copper (ii) oxide. Original molecule h2o h 2 o: But if the subscript 2 is added to make h2o2 h 2 o 2,. Copper Oxide Formula Balanced.
From www.numerade.com
SOLVEDExample Write Out the balanced chemical equation for the Copper Oxide Formula Balanced In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper (ii) oxide belongs to the monoclinic crystal system. If they weigh the reactants and products. The balanced equation will appear. The copper atom is coordinated by 4 oxygen atoms. Balanced symbol equations show what happens to the. Copper Oxide Formula Balanced.
From www.youtube.com
How to Write the Net Ionic Equation for CuO + HCl = CuCl2 + H2O YouTube Copper Oxide Formula Balanced But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. Balanced symbol equations show what happens to the different atoms in reactions. The copper atom is coordinated by 4 oxygen atoms. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. 1 atom in reagents and. Copper Oxide Formula Balanced.
From www.numerade.com
SOLVED Provide formulas, and a balanced equation for the following Copper Oxide Formula Balanced Copper (ii) oxide belongs to the monoclinic crystal system. In order to balance o. You can see that now there are two copper atoms and two oxygen atoms on each. 2cu + o 2 → 2cuo. For example, copper and oxygen react together to make. Copper + dioxygen = copper (ii) oxide. 1 atom in reagents and 2 atoms in. Copper Oxide Formula Balanced.
From www.shutterstock.com
Cuo Cupric Oxide Molecule Stock Vector (Royalty Free) 406276324 Copper Oxide Formula Balanced The balanced equation will appear. 1 atom in reagents and 1 atom in products. 2cu + o 2 → 2cuo. In order to balance o. Here is the balanced symbol equation. But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. If they weigh the reactants and products. Balanced symbol equations show what. Copper Oxide Formula Balanced.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper Oxide Formula Balanced Here is the balanced symbol equation. You can see that now there are two copper atoms and two oxygen atoms on each. 1 atom in reagents and 1 atom in products. 2cu + o 2 → 2cuo. Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole. In this experiment, students heat. Copper Oxide Formula Balanced.
From www.youtube.com
How to Balance Copper plus Oxygen Gas YouTube Copper Oxide Formula Balanced 1 atom in reagents and 2 atoms in products. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Copper (ii) oxide belongs to the monoclinic crystal system. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Cu + o2. Copper Oxide Formula Balanced.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas Copper Oxide Formula Balanced If the coefficient 2 is added in front, that makes 2 water molecules; But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. Copper + dioxygen = copper (ii) oxide. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Cu + o2 = cuo is. Copper Oxide Formula Balanced.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Oxide Formula Balanced Copper (ii) oxide belongs to the monoclinic crystal system. Original molecule h2o h 2 o: In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Here is the balanced symbol equation. Copper + dioxygen = copper (ii) oxide. 1 atom in reagents and 2 atoms in products. If. Copper Oxide Formula Balanced.
From www.numerade.com
SOLVED Consider the reaction shown. copper + oxygen → copper (I) oxide Copper Oxide Formula Balanced 1 atom in reagents and 1 atom in products. For example, copper and oxygen react together to make. 2cu + o 2 → 2cuo. Copper (ii) oxide belongs to the monoclinic crystal system. Cu + o2 = cuo is a synthesis reaction where two moles of copper [cu] and one mole. Copper + dioxygen = copper (ii) oxide. In this. Copper Oxide Formula Balanced.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Copper Oxide Formula Balanced Here is the balanced symbol equation. If they weigh the reactants and products. For example, copper and oxygen react together to make. Balanced symbol equations show what happens to the different atoms in reactions. If the coefficient 2 is added in front, that makes 2 water molecules; The balanced equation will appear. But if the subscript 2 is added to. Copper Oxide Formula Balanced.
From www.youtube.com
How to Write the Name for Cu2O Copper (I) oxide YouTube Copper Oxide Formula Balanced The copper atom is coordinated by 4 oxygen atoms. But if the subscript 2 is added to make h2o2 h 2 o 2, that's hydrogen peroxide. Copper + dioxygen = copper (ii) oxide. 1 atom in reagents and 1 atom in products. 1 atom in reagents and 2 atoms in products. Here is the balanced symbol equation. Copper (ii) oxide. Copper Oxide Formula Balanced.
From byjus.com
how to find the formula of copper oxide Copper Oxide Formula Balanced For example, copper and oxygen react together to make. Original molecule h2o h 2 o: 1 atom in reagents and 2 atoms in products. Here is the balanced symbol equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balanced symbol equations show what happens to the different atoms in reactions. The. Copper Oxide Formula Balanced.