Standard Reduction Potential Nitric Oxide . What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. You may rewrite a reaction by replacing h + with h 3 o + and. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Herein, by means of the grand canonical. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr).
from www.bartleby.com
Herein, by means of the grand canonical. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h + with h 3 o + and. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and.
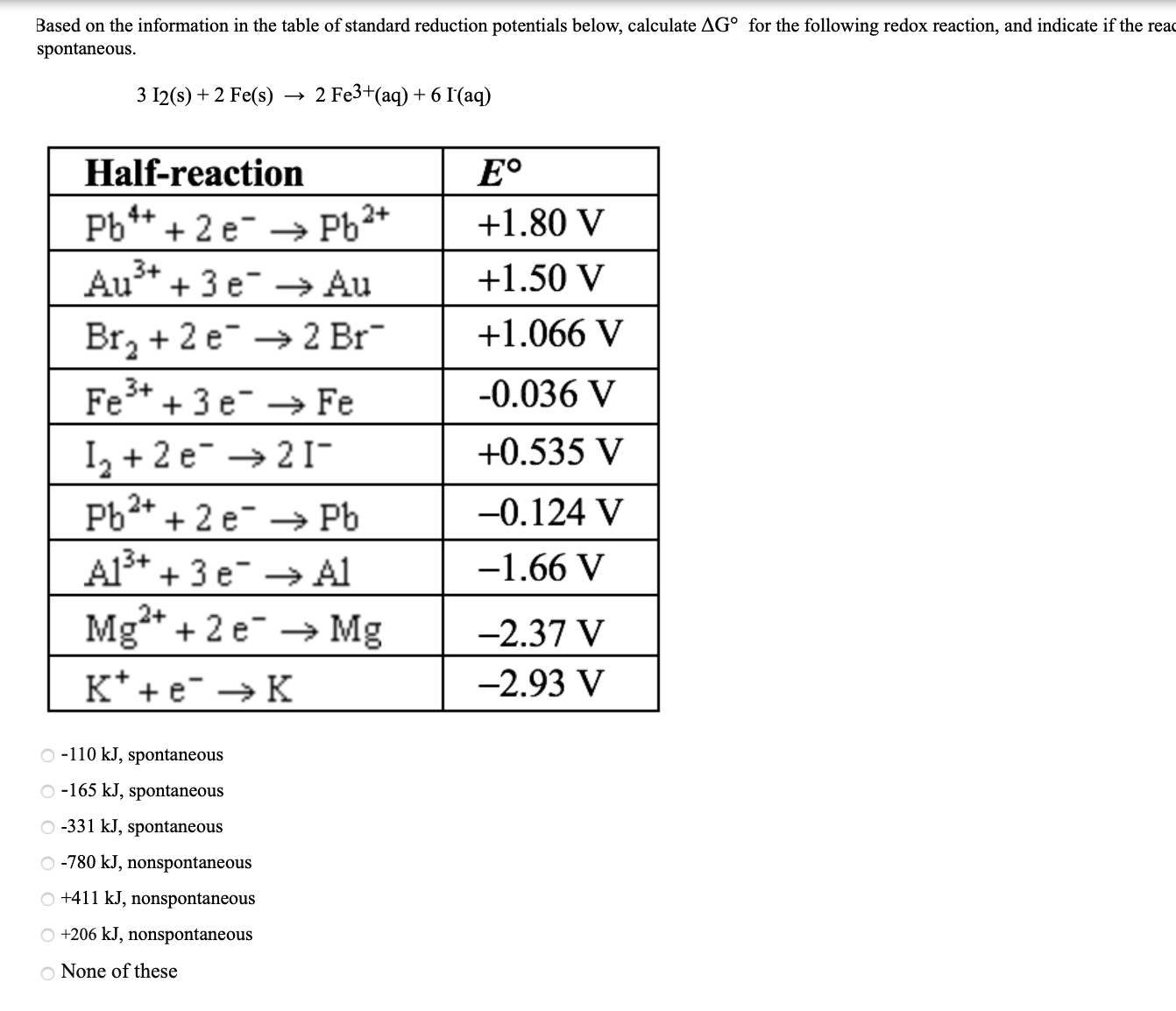
Answered Based on the information in the table… bartleby
Standard Reduction Potential Nitric Oxide You may rewrite a reaction by replacing h + with h 3 o + and. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Herein, by means of the grand canonical. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. You may rewrite a reaction by replacing h + with h 3 o + and. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). Reduction reactions in acidic solution are written using h + in place of h 3 o +.
From www.chegg.com
Solved Supposreduction of nitric oxide proceeds by the Standard Reduction Potential Nitric Oxide The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). Herein, by means of the grand canonical. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing. Standard Reduction Potential Nitric Oxide.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Nitric Oxide Herein, by means of the grand canonical. You may rewrite a reaction by replacing h + with h 3 o + and. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). The direct electrocatalytic reduction of nitrogen oxide can produce. Standard Reduction Potential Nitric Oxide.
From www.frontiersin.org
Frontiers Cyclic Conversions in the Nitrogen Cycle Standard Reduction Potential Nitric Oxide Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far. Standard Reduction Potential Nitric Oxide.
From pubs.acs.org
ChemicalPotentialDependent Thermodynamic Study of Electrochemical Standard Reduction Potential Nitric Oxide Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. Herein, by means of the grand canonical. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. You may rewrite a reaction by replacing h + with h 3 o + and. The direct electrocatalytic reduction of. Standard Reduction Potential Nitric Oxide.
From www.numerade.com
SOLVED Standard Reduction Potentials at 25^∘C^* Copyright (c) The Standard Reduction Potential Nitric Oxide What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Herein, by means of the grand canonical. Reduction reactions in acidic solution are written using h + in place. Standard Reduction Potential Nitric Oxide.
From www.chegg.com
Solved Use standard reduction potentials to calculate the Standard Reduction Potential Nitric Oxide Reduction reactions in acidic solution are written using h + in place of h 3 o +. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). You may rewrite a reaction by. Standard Reduction Potential Nitric Oxide.
From www.numerade.com
SOLVED The reaction between solid copper and nitric acid to form Standard Reduction Potential Nitric Oxide Reduction reactions in acidic solution are written using h + in place of h 3 o +. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Herein, by. Standard Reduction Potential Nitric Oxide.
From people.wou.edu
Redox Reactions Standard Reduction Potential Nitric Oxide Reduction reactions in acidic solution are written using h + in place of h 3 o +. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Herein, by means of the grand canonical. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction. Standard Reduction Potential Nitric Oxide.
From pubs.rsc.org
Electrochemical oxidation of molecular nitrogen to nitric acid Standard Reduction Potential Nitric Oxide Herein, by means of the grand canonical. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Reduction reactions in acidic solution are written using h + in place of h 3 o. Standard Reduction Potential Nitric Oxide.
From www.chegg.com
Solved Standard Electrode Potentials in Aqueous Solution at Standard Reduction Potential Nitric Oxide You may rewrite a reaction by replacing h + with h 3 o + and. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. Herein, by. Standard Reduction Potential Nitric Oxide.
From www.omicsonline.org
biological functions of major physiological Standard Reduction Potential Nitric Oxide What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction. Standard Reduction Potential Nitric Oxide.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C Standard Reduction Potential Nitric Oxide Herein, by means of the grand canonical. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). What’s more, the standard reduction potential of. Standard Reduction Potential Nitric Oxide.
From www.vrogue.co
Standard Reduction Potential Table Lydiarillynn vrogue.co Standard Reduction Potential Nitric Oxide You may rewrite a reaction by replacing h + with h 3 o + and. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. Herein, by means of the grand canonical. Reduction reactions in acidic solution are written using h. Standard Reduction Potential Nitric Oxide.
From www.studocu.com
CB Reduction Potentials STANDARD REDUCTION POTENTIALS IN AQUEOUS Standard Reduction Potential Nitric Oxide Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. Herein, by means of the grand canonical. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. You may rewrite a reaction by replacing h + with h 3 o + and. The direct electrocatalytic reduction of. Standard Reduction Potential Nitric Oxide.
From methanotroph.org
Metabolic Pathways Methanotroph Commons Standard Reduction Potential Nitric Oxide Reduction reactions in acidic solution are written using h + in place of h 3 o +. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. You may. Standard Reduction Potential Nitric Oxide.
From www.pinterest.com
Nitric oxide is a molecule that our body produces to help the entire Standard Reduction Potential Nitric Oxide Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h + with h 3 o + and. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Nitrate/nitrite +420 mv, nitrite/nitric. Standard Reduction Potential Nitric Oxide.
From www.researchgate.net
Electrochemical potentials of possible CO 2 reduction reactions in Standard Reduction Potential Nitric Oxide What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Herein, by means of the grand canonical. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). The direct electrocatalytic reduction of nitrogen oxide can produce. Standard Reduction Potential Nitric Oxide.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Nitric Oxide The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). You may rewrite a reaction by replacing h + with h 3 o + and. Herein, by means of the grand canonical. Reduction. Standard Reduction Potential Nitric Oxide.
From www.pnas.org
Ammonia oxidation pathways and nitrifier denitrification are Standard Reduction Potential Nitric Oxide You may rewrite a reaction by replacing h + with h 3 o + and. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Reduction reactions in acidic. Standard Reduction Potential Nitric Oxide.
From ch302.cm.utexas.edu
stdpotsshortlist.png Standard Reduction Potential Nitric Oxide What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. You may rewrite a reaction by replacing h + with h 3 o + and. Reduction reactions in acidic solution are written using h + in place. Standard Reduction Potential Nitric Oxide.
From www.flinnsci.com
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Nitric Oxide Reduction reactions in acidic solution are written using h + in place of h 3 o +. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. You may rewrite a reaction by replacing h + with h 3 o + and. The chemical potential (μ). Standard Reduction Potential Nitric Oxide.
From www.researchgate.net
Proposed electrochemical reduction mechanism of the Fe(III) complex on Standard Reduction Potential Nitric Oxide Herein, by means of the grand canonical. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Reduction reactions in. Standard Reduction Potential Nitric Oxide.
From www.chegg.com
Solved Suppose the reduction of nitric oxide proceeds by the Standard Reduction Potential Nitric Oxide Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). You may rewrite a reaction by replacing h + with h 3 o + and. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than. Standard Reduction Potential Nitric Oxide.
From ecampusontario.pressbooks.pub
Redox Reactions Review BIOC*2580 Introduction to Biochemistry Standard Reduction Potential Nitric Oxide What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Herein, by means of the grand canonical. The chemical potential (μ) is a substantial but ignored factor in many. Standard Reduction Potential Nitric Oxide.
From www.slideserve.com
PPT ELECTRON TRANSFER PowerPoint Presentation ID684192 Standard Reduction Potential Nitric Oxide What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Herein, by means of the grand canonical. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. The chemical potential (μ) is a substantial but ignored factor in many. Standard Reduction Potential Nitric Oxide.
From www.yumpu.com
The reduction potential of nitric oxide (NO) and its importance to NO Standard Reduction Potential Nitric Oxide Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h + with h 3 o + and. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. Herein, by means of the grand canonical. What’s more, the standard reduction potential of norr (0.71 v vs rhe). Standard Reduction Potential Nitric Oxide.
From www.researchgate.net
1 The various routes of nitric oxide formation in plants. Reductive Standard Reduction Potential Nitric Oxide Herein, by means of the grand canonical. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). You may rewrite a reaction by replacing h + with h 3 o + and. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of. Standard Reduction Potential Nitric Oxide.
From www.researchgate.net
The nitric oxide (NO), nitrite (NO2) and nitrate (NO3) cycle Standard Reduction Potential Nitric Oxide What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. Herein, by means of the grand canonical. You may rewrite. Standard Reduction Potential Nitric Oxide.
From www.researchgate.net
Standard electrochemical potentials for CO 2 reduction. Download Standard Reduction Potential Nitric Oxide Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). Reduction reactions in acidic solution are written using h + in place of h. Standard Reduction Potential Nitric Oxide.
From www.numerade.com
Selective Oxidation The standard reduction potential for the half Standard Reduction Potential Nitric Oxide What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may. Standard Reduction Potential Nitric Oxide.
From www.semanticscholar.org
Figure 5 from Nitrate Reduction to Nitrite, Nitric Oxide and Ammonia by Standard Reduction Potential Nitric Oxide You may rewrite a reaction by replacing h + with h 3 o + and. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. Herein, by. Standard Reduction Potential Nitric Oxide.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Nitric Oxide The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). Reduction reactions in acidic solution are written using h + in place of h 3 o +. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. You may rewrite a reaction by replacing h + with h 3 o +. Standard Reduction Potential Nitric Oxide.
From www.chegg.com
Solved Refer to the table of standard reduction potentials Standard Reduction Potential Nitric Oxide Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h + with h 3 o + and. The chemical potential (μ) is a substantial but ignored factor in many theoretical studies of electrochemical nitric oxide reduction (norr). Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric. Standard Reduction Potential Nitric Oxide.
From www.bartleby.com
Answered Based on the information in the table… bartleby Standard Reduction Potential Nitric Oxide Herein, by means of the grand canonical. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. You may rewrite a reaction by replacing h + with h 3 o + and. Reduction reactions in acidic solution are written using h + in place of h. Standard Reduction Potential Nitric Oxide.
From ch302.cm.utexas.edu
Standard Reduction Potentials Standard Reduction Potential Nitric Oxide The direct electrocatalytic reduction of nitrogen oxide can produce various products depending on the reaction conditions and. Nitrate/nitrite +420 mv, nitrite/nitric oxide +375 mv, nitric oxide/nitrous. What’s more, the standard reduction potential of norr (0.71 v vs rhe) is far beyond than those of n 2 reduction reaction (nrr, 0.09. The chemical potential (μ) is a substantial but ignored factor. Standard Reduction Potential Nitric Oxide.