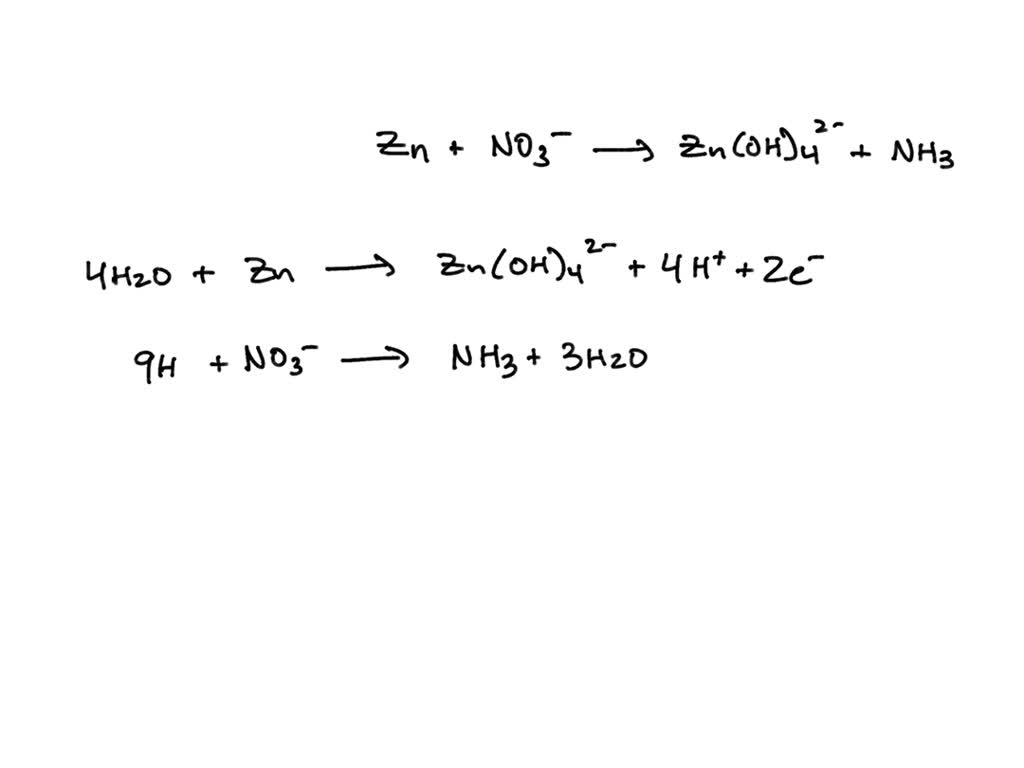Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate . The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. 2 atoms in reagents and. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Zinc is oxidized by hydrochloric acid to form zinc chloride. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zinc metal reacts with hydrochloric acid according to the balanced equation: In the process, hydrogen gas is produced. The balanced equation will appear. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. In order to balance h on both sides we: Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. The reaction is given below. Write the balanced chemical equation.
from www.numerade.com
The reaction is given below. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. 2 atoms in reagents and. In the process, hydrogen gas is produced. The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Write the balanced chemical equation. In order to balance h on both sides we: Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. The balanced equation will appear.
SOLVED Question 9 2 pts Refer to the chemical equation Zn(s) 2HCIaq
Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zinc metal reacts with hydrochloric acid according to the balanced equation: 2 atoms in reagents and. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. The reaction is given below. The balanced equation will appear. Zinc is oxidized by hydrochloric acid to form zinc chloride. In order to balance h on both sides we: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. Write the balanced chemical equation. In the process, hydrogen gas is produced.
From slideplayer.com
Chapter 8 Preview Lesson Starter Objectives ppt download Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. In the process, hydrogen gas is produced. Zinc metal reacts with hydrochloric acid according to the balanced equation: Write the balanced chemical equation. Zinc is oxidized by hydrochloric acid to form zinc chloride. In order to balance h on both sides we: 2 atoms in reagents. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.slideshare.net
Acids And Bases Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The balanced equation will appear. 2 atoms in reagents and. Zinc metal reacts with hydrochloric acid according to the balanced equation: The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. In order to balance h on both sides we: Write the balanced chemical equation. The reaction is given below. In the process, hydrogen gas is. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From warreninstitute.org
Unlock The POWER Of Chemical Reaction Zn + HCl To Zinc + Hydrochloric Acid Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Write the balanced chemical equation. The reaction is given below. In the process, hydrogen gas is produced. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Your tool of choice. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED When zinc metal is placed in solution of hydrochloric acid Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The balanced equation will appear. 2 atoms in reagents and. Write the balanced chemical equation. In order to balance h on both sides we: Zinc metal reacts with hydrochloric acid according to the balanced equation: The reaction is given below. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zn(s) + 2. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From slideplayer.com
Balancing Chemical Equations ppt download Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Write the balanced chemical equation. The balanced equation will appear. Zinc metal reacts with hydrochloric acid according to the balanced equation:. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Write the balanced equations for 1. The reaction of zinc Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Write the balanced chemical equation. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid,. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.chegg.com
Solved 1. Zinc metal reacts with hydrochloric acid according Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Write the balanced chemical equation. Zinc metal reacts with hydrochloric acid according to the balanced equation: Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: In the process, hydrogen gas is produced. Zn(s). Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate In the process, hydrogen gas is produced. 2 atoms in reagents and. In order to balance h on both sides we: The reaction is given below. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. The balanced equation will appear. Write the balanced chemical equation. To balance. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From slidetodoc.com
Chemical Equations and Reactions Chemical Reactions l A Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate In the process, hydrogen gas is produced. The reaction is given below. 2 atoms in reagents and. Write the balanced chemical equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In the process, hydrogen gas is produced. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. Zinc is oxidized by hydrochloric acid to form zinc chloride. Zinc. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Calculate the mass of hydrochloric acid (HCl) needed to react Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. In order to balance h on both sides we: Zinc is oxidized by hydrochloric acid to form zinc chloride. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The reaction is given below. Zinc metal reacts with hydrochloric acid according to the balanced equation: 2 atoms in reagents and. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. Write the balanced chemical equation. The reaction of zinc metal and hydrochloric acid produces. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVEDZinc metal reacts with hydrochloric acid according to the Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate In the process, hydrogen gas is produced. Zinc is oxidized by hydrochloric acid to form zinc chloride. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Write two balanced chemical reactions, one for the reaction of Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. Write the balanced chemical equation. Zinc metal reacts with hydrochloric acid according to the balanced equation: In order to balance h. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From express.adobe.com
Zinc and Hydrochloric Acid Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. 2 atoms in reagents and. Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc is oxidized by hydrochloric acid to form zinc chloride. Multiply coefficient for hcl by 2 1 zn +. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Question 9 2 pts Refer to the chemical equation Zn(s) 2HCIaq Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The balanced equation will appear. 2 atoms in reagents and. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. In order to. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate In order to balance h on both sides we: Zinc metal reacts with hydrochloric acid according to the balanced equation: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. 2. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From slideplayer.com
Ch. 8 Chemical Equations and Reactions ppt download Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc is oxidized by hydrochloric acid to form zinc chloride. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED The reaction of zinc metal and hydrochloric acid produces Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate In order to balance h on both sides we: Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Consider the reaction of zinc metal with hydrochloric acid, HCl Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate 2 atoms in reagents and. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. The reaction is given below. Zinc is oxidized by hydrochloric acid to form zinc chloride. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Write and balance this chemical equation zinc burns in oxygen Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Zinc is oxidized by hydrochloric acid to form zinc chloride. The reaction is. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate In the process, hydrogen gas is produced. The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. 2 atoms in reagents and. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED BALANCING CHEMICAL EQUATIONS HYDROGEN GAS OXYGEN GAS WATER Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate In the process, hydrogen gas is produced. Zinc is oxidized by hydrochloric acid to form zinc chloride. Zinc metal reacts with hydrochloric acid according to the balanced equation: Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g). Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate In order to balance h on both sides we: 2 atoms in reagents and. Write the balanced chemical equation. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zinc metal reacts with hydrochloric acid according to the balanced equation: The reaction of zinc metal and hydrochloric acid. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.slideserve.com
PPT Chapter 8 Chemical Equations and Reactions PowerPoint Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. In order to balance h on both sides we: Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. To balance a chemical. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The reaction is given below. Zinc is oxidized by hydrochloric acid to form zinc chloride. 2 atoms in reagents and. The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Write the balanced chemical equation. In order. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The reaction is given below. Write the balanced chemical equation. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Zinc is oxidized. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From hydrogenchloridemekaiga.blogspot.com
Hydrogen Chloride Zinc Hydrogen Chloride Balanced Equation Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The reaction is given below. 2 atoms in reagents and. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. Zinc metal reacts with hydrochloric acid according to the balanced equation: To balance a chemical equation, enter an equation of a chemical reaction and press. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED A 15 g block of zinc is dissolved in 1 M hydrochloric acid (a Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Write the balanced chemical equation. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: The balanced equation will appear. In the process, hydrogen gas is produced. In order to balance h on both sides we: Your tool of choice here will be the mole ratio that exists. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: In the process, hydrogen gas is produced. Write the balanced chemical equation. 2 atoms in reagents and. The reaction is given below. Zinc metal reacts with hydrochloric acid according to the balanced equation: Your tool of choice here. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.slideserve.com
PPT Ch. 8 Chemical Equations and Reactions PowerPoint Presentation Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Zn(s) + 2 hcl(aq)¡zncl2(aq) + h2( g) when 0.103 g of zn(s) is combined with enough hcl to make 50.0 ml of. The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Zinc is. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Zinc And Hydrochloric Acid To Generate Hydrogen Gas Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The balanced equation will appear. The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. The reaction is given below. Zinc is oxidized by hydrochloric acid to form zinc chloride. Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: Your tool of choice. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Similar to magnesium, zinc also reacts with hydrochloric acid Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate Multiply coefficient for hcl by 2 1 zn + 2 hcl = 1 h 2 + 1 zncl 2 cl is balanced: The reaction of zinc metal and hydrochloric acid produces hydrogen gas and zinc chloride. Zinc metal reacts with hydrochloric acid according to the balanced equation: In the process, hydrogen gas is produced. The reaction is given below. In. Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate The balanced equation will appear. Your tool of choice here will be the mole ratio that exists between zinc metal, zn, and hydrochloric acid, :hcl, in the balanced chemical equation. 2 atoms in reagents and. Write the balanced chemical equation. Zinc is oxidized by hydrochloric acid to form zinc chloride. In order to balance h on both sides we: Zn(s). Balanced Chemical Equation For Zinc And Hydrochloric Acid To Generate.