How To Tell If An Element Is Brittle . Ionic solids do not conduct electricity; The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. These are electronegative elements with high ionization energies. Silicon is a metalloid because it has luster, but is brittle. Although they are hard, they also tend to be brittle, and they shatter rather than bend.
from yenaengineering.nl
The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These are electronegative elements with high ionization energies. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Although they are hard, they also tend to be brittle, and they shatter rather than bend. Silicon is a metalloid because it has luster, but is brittle. Ionic solids do not conduct electricity;
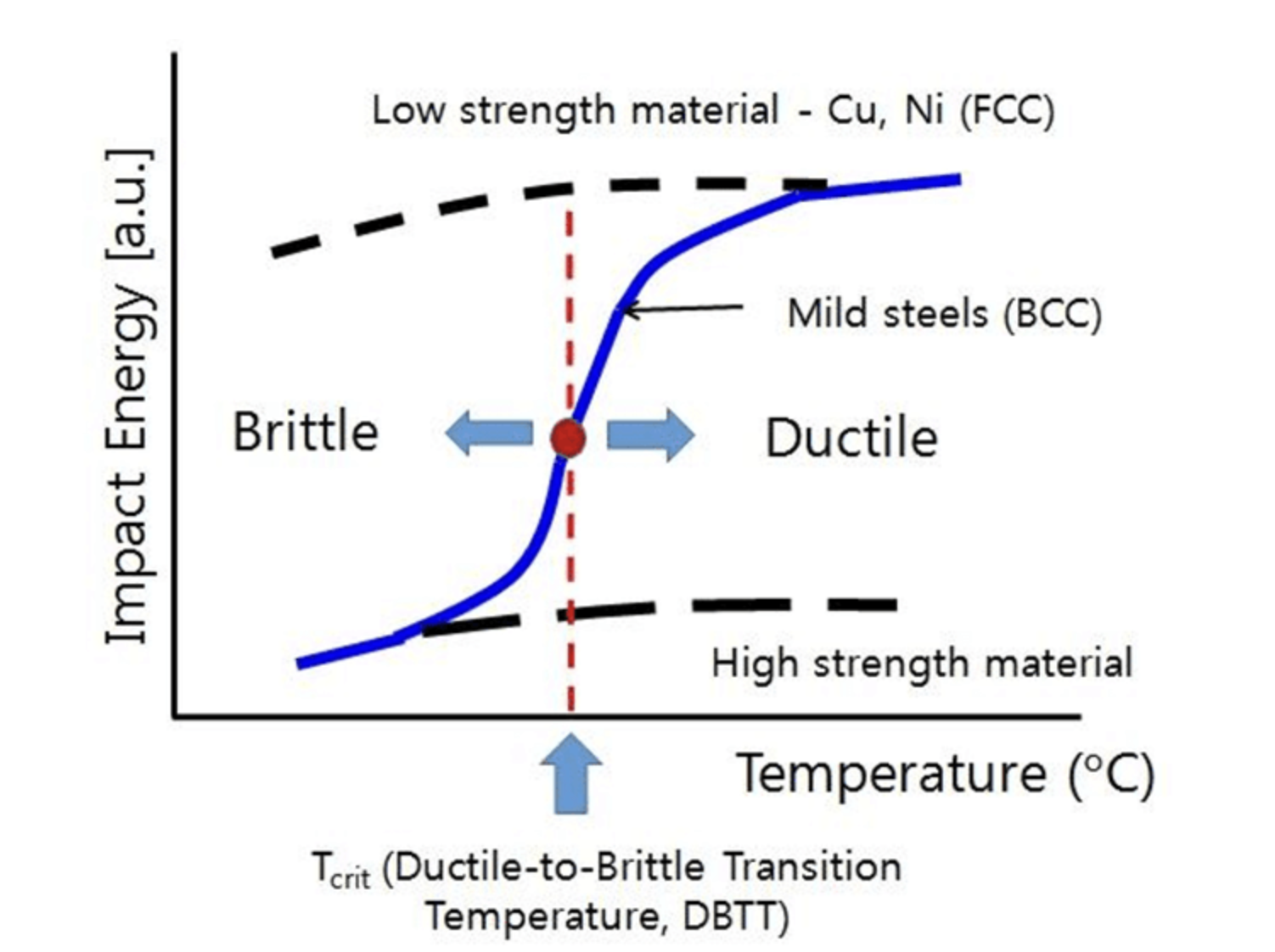
How to a Ductile to Brittle Transition Temperature
How To Tell If An Element Is Brittle Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Although they are hard, they also tend to be brittle, and they shatter rather than bend. Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Ionic solids do not conduct electricity; These are electronegative elements with high ionization energies.
From geologylearn.blogspot.com
Learning Geology Brittle Structures How To Tell If An Element Is Brittle Silicon is a metalloid because it has luster, but is brittle. Ionic solids do not conduct electricity; These are electronegative elements with high ionization energies. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Although they are hard, they also tend to be brittle, and they shatter rather than bend. Brittleness is the opposite of. How To Tell If An Element Is Brittle.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID How To Tell If An Element Is Brittle Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. These are electronegative elements with high ionization energies. Silicon is a metalloid because it has luster, but is brittle. Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line. How To Tell If An Element Is Brittle.
From slideplayer.com
History and Arrangement of the Periodic Table ppt download How To Tell If An Element Is Brittle Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Silicon is a metalloid because it has luster, but is brittle. Ionic solids do not conduct electricity; The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These are electronegative elements with high. How To Tell If An Element Is Brittle.
From simplesciencechemistry.weebly.com
Periodic Table Simple Science Chemistry How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. These are electronegative elements with high ionization energies. Although they are hard, they also tend to be brittle, and they shatter rather than. How To Tell If An Element Is Brittle.
From blog.thepipingmart.com
Is Zinc a Brittle Metal? How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not conduct electricity; Although they are hard, they also tend to be brittle, and they shatter rather than bend. Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of ductility, in which a material undergoes little to. How To Tell If An Element Is Brittle.
From slidetodoc.com
Unit 3 Periodic Table Have your Periodic Table How To Tell If An Element Is Brittle Ionic solids do not conduct electricity; Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These. How To Tell If An Element Is Brittle.
From www.slideserve.com
PPT How to Read the Periodic Table PowerPoint Presentation, free How To Tell If An Element Is Brittle Silicon is a metalloid because it has luster, but is brittle. Although they are hard, they also tend to be brittle, and they shatter rather than bend. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Ionic solids do not conduct electricity; The line begins below. How To Tell If An Element Is Brittle.
From www.slideserve.com
PPT Metals. Metalloids. Nonmetals. PowerPoint Presentation, free How To Tell If An Element Is Brittle Silicon is a metalloid because it has luster, but is brittle. Ionic solids do not conduct electricity; These are electronegative elements with high ionization energies. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. The line begins below boron (b) and extends between bismuth (bi) and. How To Tell If An Element Is Brittle.
From www.gauthmath.com
Solved The model of the periodic table below shows the locations of How To Tell If An Element Is Brittle Ionic solids do not conduct electricity; Silicon is a metalloid because it has luster, but is brittle. These are electronegative elements with high ionization energies. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Although they are hard, they also tend to be brittle, and they. How To Tell If An Element Is Brittle.
From slideplayer.com
Periodic Table Trends. ppt download How To Tell If An Element Is Brittle Although they are hard, they also tend to be brittle, and they shatter rather than bend. Ionic solids do not conduct electricity; The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These are electronegative elements with high ionization energies. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation. How To Tell If An Element Is Brittle.
From the-periodictable.weebly.com
Types of Elements the periodic table How To Tell If An Element Is Brittle Silicon is a metalloid because it has luster, but is brittle. Although they are hard, they also tend to be brittle, and they shatter rather than bend. These are electronegative elements with high ionization energies. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Ionic solids. How To Tell If An Element Is Brittle.
From www.nuclear-power.com
Ductilebrittle Transition Temperature Definition How To Tell If An Element Is Brittle These are electronegative elements with high ionization energies. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Silicon is a metalloid because it has luster, but is brittle. Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line. How To Tell If An Element Is Brittle.
From www.vrogue.co
Lewis Acids And Bases vrogue.co How To Tell If An Element Is Brittle Ionic solids do not conduct electricity; These are electronegative elements with high ionization energies. Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. The line begins below boron (b) and extends between bismuth (bi) and. How To Tell If An Element Is Brittle.
From bucarotechelp.com
Brief Description of the Chemical and Physical Properties of Elements How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not conduct electricity; Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Although they are hard, they also. How To Tell If An Element Is Brittle.
From www.gauthmath.com
Solved What type of element is brittle and acts as an insulator? Metal How To Tell If An Element Is Brittle Ionic solids do not conduct electricity; Silicon is a metalloid because it has luster, but is brittle. Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These are electronegative elements with high ionization energies. Brittleness is the opposite of. How To Tell If An Element Is Brittle.
From yenaengineering.nl
How to a Ductile to Brittle Transition Temperature How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Silicon is a metalloid because it has luster, but is brittle. These are electronegative elements with high ionization energies. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Ionic solids do not. How To Tell If An Element Is Brittle.
From www.slideshare.net
The Periodic Table How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These are electronegative elements with high ionization energies. Although they are hard, they also tend to be brittle, and they shatter rather than bend. Ionic solids do not conduct electricity; Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of. How To Tell If An Element Is Brittle.
From brainly.com
15 which element can be brittle or soft in a solid phase and is poor How To Tell If An Element Is Brittle Ionic solids do not conduct electricity; These are electronegative elements with high ionization energies. Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of. How To Tell If An Element Is Brittle.
From wagine.com
17 Metals With the Highest Melting Points (and Why) Materials Science How To Tell If An Element Is Brittle These are electronegative elements with high ionization energies. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Although they are hard,. How To Tell If An Element Is Brittle.
From www.breakingatom.com
Brittle Definition How To Tell If An Element Is Brittle Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. These are electronegative elements with high ionization energies. Silicon is a metalloid because it has luster, but is brittle. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not. How To Tell If An Element Is Brittle.
From schoolbag.info
Figure 2.3. Periodic Table, Coded by Element Type How To Tell If An Element Is Brittle Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Silicon is a metalloid because it has luster, but is brittle. These are electronegative elements with high ionization energies. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not. How To Tell If An Element Is Brittle.
From www.youtube.com
Ductile and Brittle Materials by stress strain curve YouTube How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not conduct electricity; Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Although they are hard, they also tend to be brittle, and they shatter rather than bend. These. How To Tell If An Element Is Brittle.
From sciencenotes.org
Free Printable Periodic Tables (PDF and PNG) Science Notes and Projects How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not conduct electricity; Although they are hard, they also tend to be brittle, and they shatter rather than bend. These are electronegative elements with high ionization energies. Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of. How To Tell If An Element Is Brittle.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2975896 How To Tell If An Element Is Brittle Silicon is a metalloid because it has luster, but is brittle. These are electronegative elements with high ionization energies. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Although they are hard,. How To Tell If An Element Is Brittle.
From www.mikeblaber.org
Useful Tables Atomic Periodicity How To Tell If An Element Is Brittle Silicon is a metalloid because it has luster, but is brittle. Although they are hard, they also tend to be brittle, and they shatter rather than bend. Ionic solids do not conduct electricity; Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. These are electronegative elements. How To Tell If An Element Is Brittle.
From elchoroukhost.net
Periodic Table Of Elements With Names And Symbols Elcho Table How To Tell If An Element Is Brittle Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not conduct electricity; Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Silicon. How To Tell If An Element Is Brittle.
From deborahhindi.com
Example Of A Brittle Element How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not conduct electricity; Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. These are electronegative elements with high ionization energies. Silicon is a metalloid because it has luster, but. How To Tell If An Element Is Brittle.
From www.dreamstime.com
Iridium, Ir, Periodic Table Element Stock Illustration Illustration How To Tell If An Element Is Brittle Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These are electronegative elements with high ionization energies. Silicon is a metalloid because it has luster, but is brittle. Ionic solids do not conduct electricity; Brittleness is the opposite of. How To Tell If An Element Is Brittle.
From www.youtube.com
Lesson 4 DUCTILE TO BRITTLE TRANSITION CONDITIONS YouTube How To Tell If An Element Is Brittle Silicon is a metalloid because it has luster, but is brittle. These are electronegative elements with high ionization energies. Although they are hard, they also tend to be brittle, and they shatter rather than bend. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. The line. How To Tell If An Element Is Brittle.
From slideplayer.com
Elements, Compounds, & Mixtures ppt download How To Tell If An Element Is Brittle Although they are hard, they also tend to be brittle, and they shatter rather than bend. These are electronegative elements with high ionization energies. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it. How To Tell If An Element Is Brittle.
From www.failurecriteria.com
Physical Ductility of the Elements How To Tell If An Element Is Brittle The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Ionic solids do not conduct electricity; Silicon is a metalloid because it has luster, but is brittle. Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Although they are hard, they also. How To Tell If An Element Is Brittle.
From www.slideserve.com
PPT Families of the Periodic Table PowerPoint Presentation, free How To Tell If An Element Is Brittle Ionic solids do not conduct electricity; Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it fractures. Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These. How To Tell If An Element Is Brittle.
From studyx.ai
What type of element is brittle and acts as StudyX How To Tell If An Element Is Brittle These are electronegative elements with high ionization energies. Although they are hard, they also tend to be brittle, and they shatter rather than bend. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). Brittleness is the opposite of ductility, in which a material undergoes little to no plastic deformation when under tensile stress before it. How To Tell If An Element Is Brittle.
From periodictable.com
Sample from the RGB Set, a sample of the element Tellurium in the How To Tell If An Element Is Brittle Although they are hard, they also tend to be brittle, and they shatter rather than bend. Ionic solids do not conduct electricity; Silicon is a metalloid because it has luster, but is brittle. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These are electronegative elements with high ionization energies. Brittleness is the opposite of. How To Tell If An Element Is Brittle.
From www.sscadda.com
Study notes on "MODERN PERIODIC TABLE" How To Tell If An Element Is Brittle Ionic solids do not conduct electricity; Silicon is a metalloid because it has luster, but is brittle. The line begins below boron (b) and extends between bismuth (bi) and polonium (po). These are electronegative elements with high ionization energies. Although they are hard, they also tend to be brittle, and they shatter rather than bend. Brittleness is the opposite of. How To Tell If An Element Is Brittle.