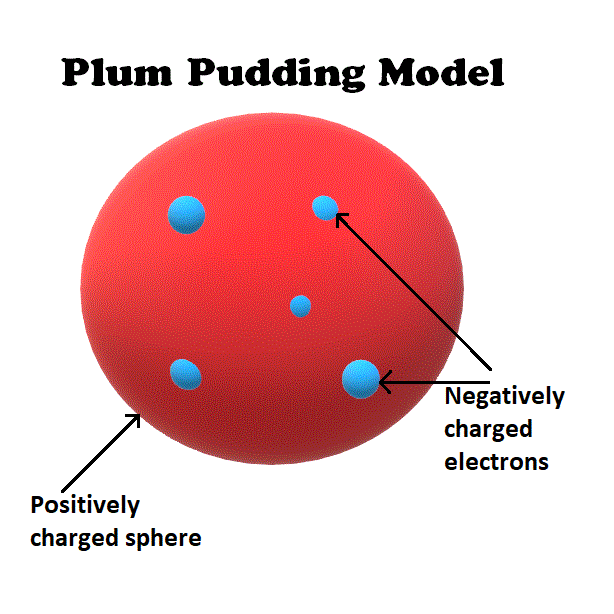
Example Plum Pudding Model . Thomson was proposed the first scientific model to explain the internal structure of an atom. Electrons are negatively charged particles,…. popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. The electrons were considered somewhat mobile. a plum pudding model is a historical scientific model of the atom that was proposed by j.j. plum pudding is an english dessert similar to a blueberry muffin. in 1898, j. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. Explore the answer through this simulation. how did rutherford figure out the structure of the atom without being able to see it? Thomson in 1904, shortly after he discovered the electron. The model tried to explain two properties of atoms that were known at that time:
from mungfali.com
popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. plum pudding is an english dessert similar to a blueberry muffin. The model tried to explain two properties of atoms that were known at that time: Thomson in 1904, shortly after he discovered the electron. how did rutherford figure out the structure of the atom without being able to see it? The electrons were considered somewhat mobile. Electrons are negatively charged particles,…. in 1898, j. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. Thomson was proposed the first scientific model to explain the internal structure of an atom.
Plum Pudding Model And Nuclear Model
Example Plum Pudding Model how did rutherford figure out the structure of the atom without being able to see it? plum pudding is an english dessert similar to a blueberry muffin. popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. Electrons are negatively charged particles,…. Thomson in 1904, shortly after he discovered the electron. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. Explore the answer through this simulation. The model tried to explain two properties of atoms that were known at that time: a plum pudding model is a historical scientific model of the atom that was proposed by j.j. The electrons were considered somewhat mobile. in 1898, j. Thomson was proposed the first scientific model to explain the internal structure of an atom. how did rutherford figure out the structure of the atom without being able to see it?
From www.slideserve.com
PPT JJ Thompson Discovery of Electrons PowerPoint Presentation Example Plum Pudding Model In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. plum pudding is an english dessert similar to a blueberry muffin. Electrons are negatively charged particles,…. Explore the answer through this simulation. Thomson in 1904, shortly after he discovered the electron. in 1898,. Example Plum Pudding Model.
From www.slideserve.com
PPT Chapter 4 Atoms PowerPoint Presentation, free download ID5528711 Example Plum Pudding Model Explore the answer through this simulation. in 1898, j. The model tried to explain two properties of atoms that were known at that time: popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a. Example Plum Pudding Model.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID151803 Example Plum Pudding Model Thomson was proposed the first scientific model to explain the internal structure of an atom. Explore the answer through this simulation. plum pudding is an english dessert similar to a blueberry muffin. how did rutherford figure out the structure of the atom without being able to see it? in 1898, j. In thomson's plum pudding model of. Example Plum Pudding Model.
From www.youtube.com
Thomson’s Plum Pudding Model of The Atom GCSE Chemistry (91 Example Plum Pudding Model plum pudding is an english dessert similar to a blueberry muffin. Explore the answer through this simulation. a plum pudding model is a historical scientific model of the atom that was proposed by j.j. how did rutherford figure out the structure of the atom without being able to see it? The electrons were considered somewhat mobile. . Example Plum Pudding Model.
From www.teachoo.com
Plum Pudding Model of Atom (JJ Thomson's Model) Postulates, Limitati Example Plum Pudding Model Thomson in 1904, shortly after he discovered the electron. Explore the answer through this simulation. plum pudding is an english dessert similar to a blueberry muffin. Thomson was proposed the first scientific model to explain the internal structure of an atom. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive. Example Plum Pudding Model.
From www.expii.com
Plum Pudding Model — Overview & Importance Expii Example Plum Pudding Model plum pudding is an english dessert similar to a blueberry muffin. in 1898, j. The electrons were considered somewhat mobile. The model tried to explain two properties of atoms that were known at that time: In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into. Example Plum Pudding Model.
From www.teachoo.com
Plum Pudding Model of Atom (JJ Thomson's Model) Postulates, Limitati Example Plum Pudding Model how did rutherford figure out the structure of the atom without being able to see it? Thomson in 1904, shortly after he discovered the electron. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. Thomson was proposed the first scientific model to explain. Example Plum Pudding Model.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID5481058 Example Plum Pudding Model plum pudding is an english dessert similar to a blueberry muffin. how did rutherford figure out the structure of the atom without being able to see it? In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. Electrons are negatively charged particles,…. . Example Plum Pudding Model.
From slideplayer.com
Plum Pudding Model. ppt download Example Plum Pudding Model in 1898, j. plum pudding is an english dessert similar to a blueberry muffin. Electrons are negatively charged particles,…. The electrons were considered somewhat mobile. Explore the answer through this simulation. Thomson was proposed the first scientific model to explain the internal structure of an atom. how did rutherford figure out the structure of the atom without. Example Plum Pudding Model.
From www.expii.com
Plum Pudding Model — Overview & Importance Expii Example Plum Pudding Model how did rutherford figure out the structure of the atom without being able to see it? Electrons are negatively charged particles,…. Explore the answer through this simulation. In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. popularly known as the plum pudding. Example Plum Pudding Model.
From www.breakingatom.com
Plum Pudding Model Definition Example Plum Pudding Model plum pudding is an english dessert similar to a blueberry muffin. Thomson was proposed the first scientific model to explain the internal structure of an atom. popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits. Example Plum Pudding Model.
From variosmodelo.blogspot.com
Describe Thomsons Plum Pudding Model Of The Atom Vários Modelos Example Plum Pudding Model In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. Electrons are negatively charged particles,…. The model tried to explain two properties of atoms that were known at that time: Thomson in 1904, shortly after he discovered the electron. popularly known as the plum. Example Plum Pudding Model.
From www.slideserve.com
PPT Development of Atomic Models PowerPoint Presentation, free Example Plum Pudding Model popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. how did rutherford figure out the structure of the atom without being able to see it? Explore the answer through this. Example Plum Pudding Model.
From www.slideserve.com
PPT RUTHERFORD’S EXPERIMENT PowerPoint Presentation, free download Example Plum Pudding Model Thomson was proposed the first scientific model to explain the internal structure of an atom. plum pudding is an english dessert similar to a blueberry muffin. Explore the answer through this simulation. a plum pudding model is a historical scientific model of the atom that was proposed by j.j. The model tried to explain two properties of atoms. Example Plum Pudding Model.
From www.youtube.com
JJ Thomson's Plum Pudding Model YouTube Example Plum Pudding Model Thomson in 1904, shortly after he discovered the electron. a plum pudding model is a historical scientific model of the atom that was proposed by j.j. how did rutherford figure out the structure of the atom without being able to see it? in 1898, j. plum pudding is an english dessert similar to a blueberry muffin.. Example Plum Pudding Model.
From mmerevise.co.uk
The History of Atomic Structure Questions and Revision MME Example Plum Pudding Model in 1898, j. Thomson in 1904, shortly after he discovered the electron. a plum pudding model is a historical scientific model of the atom that was proposed by j.j. Electrons are negatively charged particles,…. Explore the answer through this simulation. Thomson was proposed the first scientific model to explain the internal structure of an atom. In thomson's plum. Example Plum Pudding Model.
From www.youtube.com
Plum pudding model meaning what is plum pudding model what does Example Plum Pudding Model popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. in 1898, j. The electrons were considered somewhat mobile. plum pudding is an english dessert similar to a blueberry muffin.. Example Plum Pudding Model.
From www.slideserve.com
PPT History of Atomic Theory PowerPoint Presentation, free download Example Plum Pudding Model The electrons were considered somewhat mobile. The model tried to explain two properties of atoms that were known at that time: Explore the answer through this simulation. Thomson in 1904, shortly after he discovered the electron. plum pudding is an english dessert similar to a blueberry muffin. in 1898, j. a plum pudding model is a historical. Example Plum Pudding Model.
From www.youtube.com
Rutherford Scattering and the Plum Pudding ModelPhET Simulation Example Plum Pudding Model Thomson in 1904, shortly after he discovered the electron. Explore the answer through this simulation. in 1898, j. a plum pudding model is a historical scientific model of the atom that was proposed by j.j. Thomson was proposed the first scientific model to explain the internal structure of an atom. Electrons are negatively charged particles,…. The electrons were. Example Plum Pudding Model.
From mungfali.com
Plum Pudding Model And Nuclear Model Example Plum Pudding Model a plum pudding model is a historical scientific model of the atom that was proposed by j.j. how did rutherford figure out the structure of the atom without being able to see it? In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin.. Example Plum Pudding Model.
From slideplayer.com
Plum Pudding Model. ppt download Example Plum Pudding Model In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. Thomson in 1904, shortly after he discovered the electron. popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic. Example Plum Pudding Model.
From www.nuclear-power.com
Thomson Model of the Atom Plum Pudding Model Example Plum Pudding Model The model tried to explain two properties of atoms that were known at that time: In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. The electrons were considered somewhat mobile. popularly known as the plum pudding model, it had to be abandoned (1911). Example Plum Pudding Model.
From www.preparatorychemistry.com
History of Atomic Theory Example Plum Pudding Model Explore the answer through this simulation. plum pudding is an english dessert similar to a blueberry muffin. The model tried to explain two properties of atoms that were known at that time: Thomson in 1904, shortly after he discovered the electron. The electrons were considered somewhat mobile. in 1898, j. a plum pudding model is a historical. Example Plum Pudding Model.
From getrecipecart.com
Traditional plum pudding Recipe Cart Example Plum Pudding Model In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. Thomson in 1904, shortly after he discovered the electron. how did rutherford figure out the structure of the atom without being able to see it? The electrons were considered somewhat mobile. a plum. Example Plum Pudding Model.
From www.visionlearning.com
Atomic Theory I Chemistry Visionlearning Example Plum Pudding Model Thomson in 1904, shortly after he discovered the electron. Electrons are negatively charged particles,…. popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. Explore the answer through this simulation. how. Example Plum Pudding Model.
From variosmodelo.blogspot.com
Plum Pudding Model Of The Atom Vários Modelos Example Plum Pudding Model The model tried to explain two properties of atoms that were known at that time: Thomson was proposed the first scientific model to explain the internal structure of an atom. plum pudding is an english dessert similar to a blueberry muffin. The electrons were considered somewhat mobile. in 1898, j. popularly known as the plum pudding model,. Example Plum Pudding Model.
From www.slideserve.com
PPT Chapter 3 Chemical Foundations elements, atoms, and ions Example Plum Pudding Model In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. a plum pudding model is a historical scientific model of the atom that was proposed by j.j. how did rutherford figure out the structure of the atom without being able to see it?. Example Plum Pudding Model.
From revoluntaryscience.weebly.com
Atomic Theory Science Example Plum Pudding Model Electrons are negatively charged particles,…. popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. Thomson in 1904, shortly after he discovered the electron. plum pudding is an english dessert similar. Example Plum Pudding Model.
From studylib.net
Thomson, Plum Pudding Model, & Rutherford notes Example Plum Pudding Model Electrons are negatively charged particles,…. Thomson in 1904, shortly after he discovered the electron. The electrons were considered somewhat mobile. plum pudding is an english dessert similar to a blueberry muffin. Thomson was proposed the first scientific model to explain the internal structure of an atom. popularly known as the plum pudding model, it had to be abandoned. Example Plum Pudding Model.
From es.slideshare.net
JJ Thomson The Plum Pudding Model Example Plum Pudding Model popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. in 1898, j. Explore the answer through this simulation. In thomson's plum pudding model of the atom, the electrons were embedded. Example Plum Pudding Model.
From www.tes.com
Plum Pudding Model Teaching Resources Example Plum Pudding Model plum pudding is an english dessert similar to a blueberry muffin. Thomson in 1904, shortly after he discovered the electron. The electrons were considered somewhat mobile. Electrons are negatively charged particles,…. Thomson was proposed the first scientific model to explain the internal structure of an atom. In thomson's plum pudding model of the atom, the electrons were embedded in. Example Plum Pudding Model.
From recipeler.com
why is it called the plum pudding model Example Plum Pudding Model The electrons were considered somewhat mobile. Thomson was proposed the first scientific model to explain the internal structure of an atom. The model tried to explain two properties of atoms that were known at that time: Electrons are negatively charged particles,…. popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental. Example Plum Pudding Model.
From www.zmescience.com
The Plum Pudding Model how a flawed idea was instrumental in our Example Plum Pudding Model The model tried to explain two properties of atoms that were known at that time: Electrons are negatively charged particles,…. popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. how. Example Plum Pudding Model.
From www.scienceabc.com
What Is JJ Thomson's Plum Pudding Model? Example Plum Pudding Model Explore the answer through this simulation. how did rutherford figure out the structure of the atom without being able to see it? In thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. The model tried to explain two properties of atoms that were known. Example Plum Pudding Model.
From studylib.net
“Plum Pudding” model in class Bohr's Model Example Plum Pudding Model popularly known as the plum pudding model, it had to be abandoned (1911) on both theoretical and experimental grounds in favour of the rutherford atomic model, in which the electrons describe orbits about a tiny positive nucleus. Thomson in 1904, shortly after he discovered the electron. how did rutherford figure out the structure of the atom without being. Example Plum Pudding Model.