Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation . the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how to write net ionic equations for reactions that occur in aqueous solution.
from www.youtube.com
learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. learn how to write net ionic equations for reactions that occur in aqueous solution.
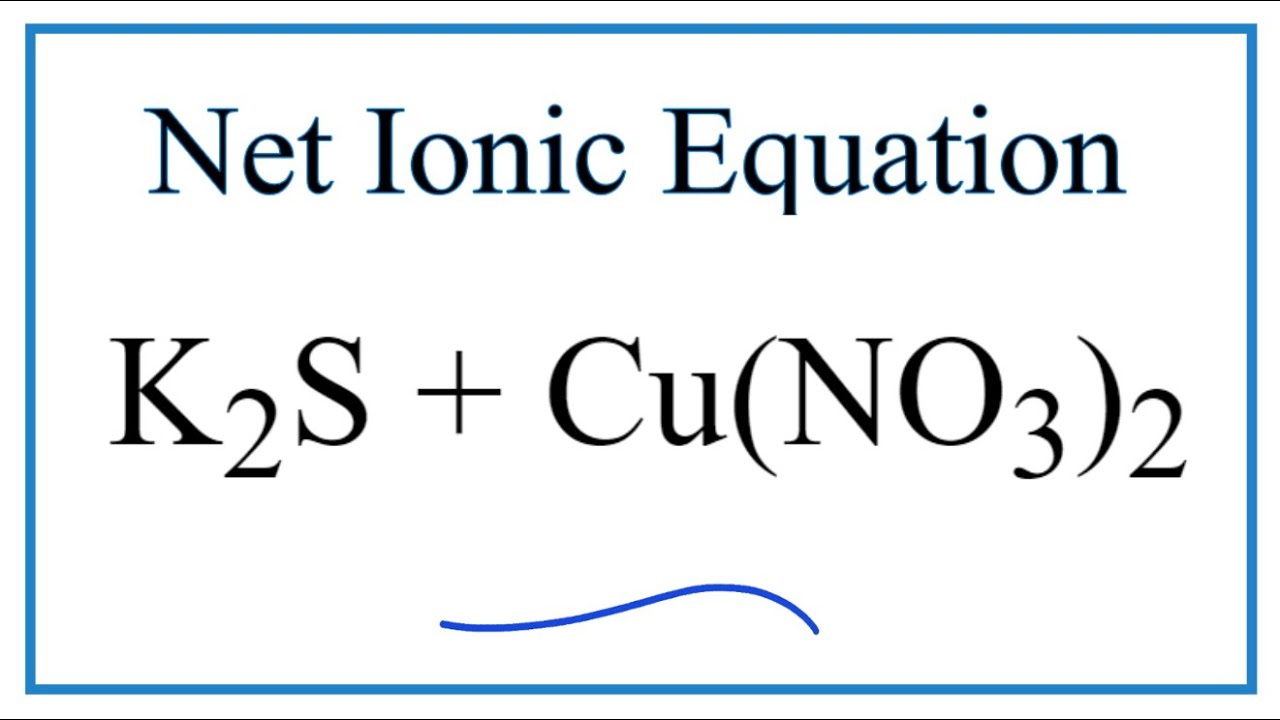
How to Write the Net Ionic Equation for K2S + Cu(NO3)2 = KNO3 + CuS
Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to write net ionic equations for reactions that occur in aqueous solution.
From printablelibpathos.z13.web.core.windows.net
Net Ionic Equation Examples With Answers Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write net ionic equations for reactions that occur in aqueous solution. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. the reaction will produce copper (ii) hydroxide, cu(oh)2,. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for K2S + Cu(NO3)2 = KNO3 + CuS Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write net ionic equations for reactions that occur in aqueous solution. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. the reaction will produce copper (ii) hydroxide,. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.wikihow.com
How to Write a Net Ionic Equation 10 Steps (with Pictures) Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. the reaction. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved 1). Select the net ionic equation for the reaction Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.slideshare.net
Chemical equations Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write net ionic equations for reactions that occur in aqueous solution. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. enter an ionic equation and get its. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reaction of Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write net ionic equations for reactions that occur in aqueous solution. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to write and. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.slideserve.com
PPT Reactions Involving Ions Molecular vs. Ionic Equations Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. the reaction. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. learn how to write. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write net ionic equations for reactions that occur in aqueous solution. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. learn how to write. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved What is the net ionic equation for Copper (ii) Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From oneclass.com
OneClass Write the balanced net ionic equation for the reactions that Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved When copper (II) chloride reacts with sodium nitrate, Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. . Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved 3. Copper (II) nitrate + Sodium phosphate Molecular Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for NiCl2 + Na2CO3 = NiCO3 + NaCl Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how to write net ionic equations for reactions that occur in aqueous solution. the reaction will produce copper. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. . Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.onlinemathlearning.com
Writing Ionic Equation (video lessons, examples and solutions) Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write net ionic equations for reactions that occur in aqueous solution. the reaction will produce copper (ii) hydroxide, cu(oh)2,. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.numerade.com
SOLVED a. What is the net ionic equation for silver nitrate + calcium Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.numerade.com
SOLVED Choose the correct net ionic equation of acetic acid being Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. enter an. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.numerade.com
SOLVED 1. Write the balanced chemical equation for the reaction of Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. use. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved What is the net ionic equation for the reaction of Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.youtube.com
2. Copper Nitrate to Copper Hydroxide (Cu Again Lab) YouTube Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write net ionic equations for reactions that occur in aqueous solution. enter an ionic equation and get. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write net ionic equations for reactions that occur in aqueous solution. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to balance the chemical equation. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.numerade.com
SOLVED Give the complete ionic equation for the reaction (if any) that Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. learn how to. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved Aqueous solutions of copper (II) nitrate and sodium Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how to write net ionic equations for reactions that occur in aqueous solution. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write and. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.chegg.com
Solved Write the net ionic equation for the reaction between Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. the reaction will. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write net ionic equations for reactions that occur in aqueous solution. enter an ionic equation and get its complete. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. learn how to write net ionic equations for reactions that occur in aqueous solution. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. use either a net ionic equations (omit. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to write net ionic equations for reactions that occur in aqueous solution. learn how to write. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 = CuO + H2O YouTube Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to write net ionic equations for reactions that occur in aqueous solution. the reaction will produce copper (ii) hydroxide, cu(oh)2, an insoluble ionic compound that precipitates out of solution, and aqueous. learn how to balance the. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write net ionic equations for reactions that occur in aqueous solution. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. learn how to write and balance net ionic equations for precipitation reactions, such as the formation of copper. enter an ionic equation and get its. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From solvedlib.com
Write net ionic equations for the reaction, if any, t… SolvedLib Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation learn how to write net ionic equations for reactions that occur in aqueous solution. learn how to balance the chemical equation for the double replacement reaction of copper (ii) nitrate and sodium hydroxide. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write and balance. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.
From www.youtube.com
How to Write the Formula for Copper (II) nitrate YouTube Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. use either a net ionic equations (omit the na+), molecular equation (include the copper compound full) or. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. learn how to write. Copper Ii Nitrate Sodium Hydroxide Net Ionic Equation.