Ionic Equation For Lead(Ii) Nitrate Dissolving In Water . The balanced equation will be calculated along with the. Other examples of dissolution equations for. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The ionic form of the dissolution equation is our first example of an ionic equation. A solution of lead(ii) nitrate is. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Complete the balanced overall ionic equation for the mixing of these two solutions. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. For example, when nacl(aq) reacts with agno Enter an equation of an ionic chemical equation and press the balance button. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions.
from www.slideserve.com
The ionic form of the dissolution equation is our first example of an ionic equation. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. Complete the balanced overall ionic equation for the mixing of these two solutions. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Other examples of dissolution equations for. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. A solution of lead(ii) nitrate is. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Enter an equation of an ionic chemical equation and press the balance button.
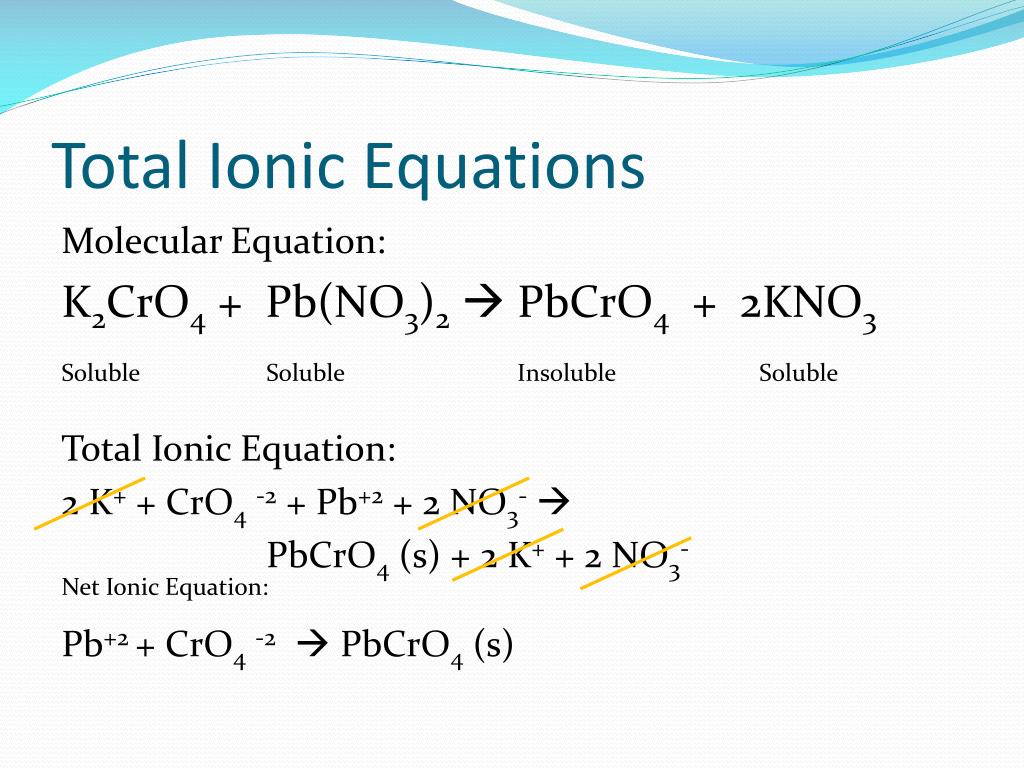
PPT Solubility and Net Ionic Equations PowerPoint Presentation, free
Ionic Equation For Lead(Ii) Nitrate Dissolving In Water A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. For example, when nacl(aq) reacts with agno The balanced equation will be calculated along with the. The ionic form of the dissolution equation is our first example of an ionic equation. Complete the balanced overall ionic equation for the mixing of these two solutions. A solution of lead(ii) nitrate is. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Enter an equation of an ionic chemical equation and press the balance button. Other examples of dissolution equations for.
From wisc.pb.unizin.org
Solutions and Solubility (part 2) (M3Q2) UWMadison Chemistry 103/104 Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Complete the balanced overall ionic equation for the mixing of these two solutions. For example, when nacl(aq) reacts with agno Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: The ionic form of the dissolution equation is our first example. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Write a balanced net ionic equation for the reaction of aqueous Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Other examples of dissolution equations for. A solution of lead(ii) nitrate is. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Enter an equation of an ionic chemical equation and press the balance button. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. This is the balanced overall ionic equation for lead(ii). Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.youtube.com
Precipitation Reactions & Net Ionic Equations Chemistry YouTube Ionic Equation For Lead(Ii) Nitrate Dissolving In Water A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. A solution of lead(ii) nitrate is. Complete the balanced overall ionic equation for the mixing of these two solutions. For example, when nacl(aq) reacts with. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.chegg.com
Solved ne Dalanced overall ionic equation for lead(Il) Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Complete the balanced overall ionic equation for the mixing of these two solutions. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. A solution of lead(ii) nitrate is. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: The. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From germanunioncemetery.org
SOLVED The Following Molecular Equation Represents The Ionic Equation For Lead(Ii) Nitrate Dissolving In Water This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: The balanced equation will be calculated along with the. Complete the balanced overall ionic equation for the mixing of these two solutions. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Solubility rules are very useful. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.youtube.com
Write the balanced chemical equation for each of the following Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. For example, when nacl(aq) reacts with agno A solution of lead(ii) nitrate is. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: Complete the balanced overall ionic equation for the. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reaction of Ionic Equation For Lead(Ii) Nitrate Dissolving In Water The ionic form of the dissolution equation is our first example of an ionic equation. The balanced equation will be calculated along with the. Other examples of dissolution equations for. Complete the balanced overall ionic equation for the mixing of these two solutions. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.slideserve.com
PPT Solubility and Net Ionic Equations PowerPoint Presentation, free Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Complete the balanced overall ionic equation for the mixing of these two solutions. For example, when nacl(aq) reacts with agno Other examples of dissolution equations for. Enter an equation of an ionic chemical equation and press the. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.chegg.com
Solved Complete the balanced overall ionic equation for Ionic Equation For Lead(Ii) Nitrate Dissolving In Water A solution of lead(ii) nitrate is. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. This is the balanced overall ionic equation for lead(ii) nitrate dissolving. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: Complete the balanced overall ionic equation for the mixing of these two solutions. A solution of lead(ii) nitrate is. Other examples of dissolution equations for. The balanced equation will be calculated along with the. A complete ionic equation is a. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From finwise.edu.vn
Albums 105+ Pictures Lead(ii) Nitrate And Sodium Iodide Superb Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Other examples of dissolution equations for. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: Lead(ii) chloride, a. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED 24) Aqueous sodium chloride and lead (II) nitrate solutions Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Enter an equation of an ionic chemical equation and press the balance button. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. A solution of lead(ii) nitrate is. Solubility rules are very useful in determining which ionic compounds are dissolved and which are. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVEDLead(II) nitrate reacts with cesium sulfate in an aqueous Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: For example, when nacl(aq) reacts with agno Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Solubility rules are very useful in determining which ionic compounds are dissolved and. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Ionic Equation For Lead(Ii) Nitrate Dissolving In Water The balanced equation will be calculated along with the. Complete the balanced overall ionic equation for the mixing of these two solutions. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: The ionic form of the dissolution. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Lead(II) nitrate reacts with cesium sulfate in an aqueous Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Other examples of dissolution equations for. Complete the balanced overall ionic equation for the mixing of these two solutions. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. For example, when nacl(aq) reacts with agno The ionic form of the dissolution equation is our first example. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED The net ionic equation for the reaction of aqueous sodium Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Other examples of dissolution equations for. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. The balanced equation will be calculated along with the. Complete the balanced overall ionic equation for the mixing of. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.chegg.com
Solved Net lonic Equations 1. silver nitrate +lead (II) Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Complete the balanced overall ionic equation for the mixing of these two solutions. Enter an equation of an ionic chemical equation and press the balance button. Solubility rules are very useful in determining which ionic compounds are dissolved and which. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED If you dissolve lead (II) nitrate and potassium iodide in water Ionic Equation For Lead(Ii) Nitrate Dissolving In Water A solution of lead(ii) nitrate is. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Other examples of dissolution equations for. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. This is the balanced overall ionic. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Write the net ionic equation for the reaction of lead(II Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Enter an equation of an ionic chemical equation and press the balance button. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Mixing aqueous lead (II) nitrate with aqueous sodium bromide Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Enter an equation of an ionic chemical equation and press the balance button. Complete the balanced overall ionic equation for the mixing of these two solutions. The balanced equation will be calculated along with the. Other examples. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.slideserve.com
PPT Ionic Equations PowerPoint Presentation, free download ID919487 Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Enter an equation of an ionic chemical equation and press the balance button. A solution of lead(ii) nitrate is. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: Complete the balanced overall ionic. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Ionic Equation For Lead(Ii) Nitrate Dissolving In Water For example, when nacl(aq) reacts with agno Enter an equation of an ionic chemical equation and press the balance button. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. The balanced equation will be calculated along with the. Complete the balanced overall ionic equation for the mixing of these two. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Enter an equation of an ionic chemical equation and press the balance button. Complete the balanced overall ionic equation for the mixing of these two solutions. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. The balanced equation will be calculated along with the. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. This is. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.chegg.com
Solved Complete the balanced overall ionic equation for Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. A solution of lead(ii) nitrate is. Other examples of dissolution equations for. For example, when nacl(aq) reacts with agno The ionic form of the dissolution equation is our first example of an ionic equation. The balanced equation will be calculated along with the. Pb(no₃)₂(s). Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Solid nickel reacts with aqueous lead (II) nitrate to form Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Enter an equation of an ionic chemical equation and press the balance button. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Solubility rules are very useful in determining which. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.slideshare.net
C20 Review Unit 02 Chemical Reactions Ionic Equation For Lead(Ii) Nitrate Dissolving In Water The ionic form of the dissolution equation is our first example of an ionic equation. The balanced equation will be calculated along with the. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Other examples of dissolution equations for. This is the balanced overall ionic equation for lead(ii) nitrate. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Complete the balanced overall ionic equation for the mixing of these two solutions. The ionic form of the dissolution equation is our first example of an ionic equation. Other examples of dissolution equations for. For example, when nacl(aq) reacts with agno Enter an equation of an ionic chemical equation and press the balance button. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.chegg.com
Solved Complete The Balanced Overall Ionic Equation For L... Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Enter an equation of an ionic chemical equation and press the balance button. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii). Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead (II) nitrate react Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Enter an equation of an ionic chemical equation and press the balance button. For example, when nacl(aq) reacts with agno The balanced equation will be calculated along with the. Other examples of dissolution equations for. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. This is the balanced overall. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.bartleby.com
Answered Many ionic compounds are water soluble,… bartleby Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. For example, when nacl(aq) reacts with agno Complete the balanced overall ionic equation for the mixing of these two solutions. The ionic form of the dissolution equation is our first example of an ionic equation. A solution of lead(ii) nitrate. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Complete the balanced overall ionic equation for the mixing of these two solutions. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. Other examples of dissolution equations for. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. The ionic. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVED Write the balanced net ionic equation for the reaction of Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Enter an equation of an ionic chemical equation and press the balance button. Complete the balanced overall ionic equation for the mixing of these two solutions. The ionic form of the dissolution equation is our first example of an ionic equation. This is the balanced overall ionic equation for lead(ii) nitrate dissolving in water: A solution of lead(ii) nitrate is.. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.youtube.com
lead (II) nitrate And Sodium Iodide Make Lead (II) iodide And sodium Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Other examples of dissolution equations for. A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. The ionic form of the dissolution equation is our first example of an ionic equation. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. Enter an equation of an ionic chemical equation and press the balance button.. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.numerade.com
SOLVEDFor each problem below, predict the products assuming double Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. Other examples of dissolution equations for. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. The balanced equation will be calculated along with the. A solution of lead(ii) nitrate is. This is the. Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.
From www.solutionspile.com
[Solved] The equation for the dissolving of lead (II) Ionic Equation For Lead(Ii) Nitrate Dissolving In Water Solubility rules are very useful in determining which ionic compounds are dissolved and which are not. Pb(no₃)₂(s) → pb₂⁺(aq) + 2no₃⁻(aq) what does. Enter an equation of an ionic chemical equation and press the balance button. Lead(ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead(ii) nitrate solution. A solution of lead(ii). Ionic Equation For Lead(Ii) Nitrate Dissolving In Water.