Titration Experiment With Acid . a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. The reagent (titrant) is the solution with a known molarity that will react with the analyte. students should be able to: The concentration of the naoh solution is. The analyte (titrand) is the solution with an unknown molarity. in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. Determine the concentration of analyte present, as well as the acid ionization constant and. By measuring the temperature change each time a portion of acid is. in this experiment, students titrate sodium hydroxide solution with hydrochloric acid.
from www.chegg.com
in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. Determine the concentration of analyte present, as well as the acid ionization constant and. in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. The concentration of the naoh solution is. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. By measuring the temperature change each time a portion of acid is. The analyte (titrand) is the solution with an unknown molarity. students should be able to:
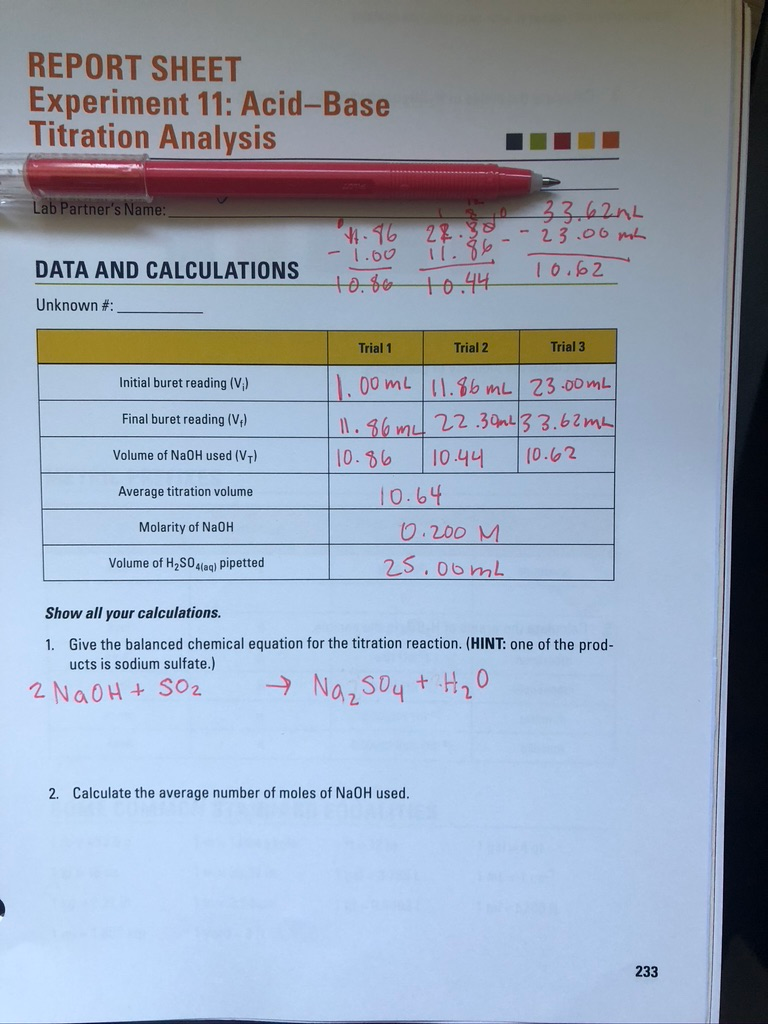
Solved REPORT SHEET Experiment 11 AcidBase Titration
Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. The analyte (titrand) is the solution with an unknown molarity. in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. Determine the concentration of analyte present, as well as the acid ionization constant and. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The concentration of the naoh solution is. By measuring the temperature change each time a portion of acid is. students should be able to: in this experiment, students titrate sodium hydroxide solution with hydrochloric acid.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Experiment With Acid The analyte (titrand) is the solution with an unknown molarity. in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations. Titration Experiment With Acid.
From fineartamerica.com
Acidbase Titration by Science Source Titration Experiment With Acid By measuring the temperature change each time a portion of acid is. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. students should be able to: a titration. Titration Experiment With Acid.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Experiment With Acid By measuring the temperature change each time a portion of acid is. Determine the concentration of analyte present, as well as the acid ionization constant and. in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. The reagent (titrant) is the solution with a known molarity that will react with the analyte.. Titration Experiment With Acid.
From mavink.com
Acid Base Titration Lab Procedure Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. By measuring the. Titration Experiment With Acid.
From www.chegg.com
Solved REPORT SHEET Experiment 11 AcidBase Titration Titration Experiment With Acid The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations. Titration Experiment With Acid.
From www.science-revision.co.uk
Titrations Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. The analyte (titrand) is the solution with an unknown molarity. By measuring the temperature change each time a portion of acid is. Describe how to carry out titrations using strong. Titration Experiment With Acid.
From www.sliderbase.com
Titration Titration Experiment With Acid The analyte (titrand) is the solution with an unknown molarity. students should be able to: in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Describe how to carry out titrations. Titration Experiment With Acid.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Titration Experiment With Acid The analyte (titrand) is the solution with an unknown molarity. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. The reagent (titrant) is the solution with a known molarity that. Titration Experiment With Acid.
From www.alamy.com
Titration experiment to measure the volume of acid needed to neutralize Titration Experiment With Acid Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. a titration is an experiment where a volume of a solution of known concentration is added to a volume of. Titration Experiment With Acid.
From www.dreamstime.com
The Chemist Adds Solutions with Precision, Ensuring Accurate Titration Experiment With Acid a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. By measuring the temperature change each time a portion of acid is. The reagent (titrant) is the solution with a known molarity that will react with the analyte. students should be able to:. Titration Experiment With Acid.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. students should be able to: Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³. Titration Experiment With Acid.
From dxokhzpxh.blob.core.windows.net
Aim Of Acid Base Titration Experiment at Ebony Mooney blog Titration Experiment With Acid students should be able to: The analyte (titrand) is the solution with an unknown molarity. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The concentration of the naoh solution is. By measuring the temperature change each time a portion of acid. Titration Experiment With Acid.
From www.youtube.com
Lab Demonstration Acid Base Titration. YouTube Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. The analyte (titrand) is the solution with an unknown molarity. in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. The concentration of the naoh solution is. Describe how to carry out titrations using strong acids and strong alkalis only. Titration Experiment With Acid.
From sciencestruck.com
Titration of Sulfuric Acid and Sodium Hydroxide Titration Experiment With Acid By measuring the temperature change each time a portion of acid is. students should be able to: a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Determine the concentration of analyte present, as well as the acid ionization constant and. in. Titration Experiment With Acid.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Experiment With Acid a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The concentration of the naoh solution is. By measuring the temperature change each time a portion of acid is. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric. Titration Experiment With Acid.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Experiment With Acid Determine the concentration of analyte present, as well as the acid ionization constant and. By measuring the temperature change each time a portion of acid is. in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a titration is an experiment. Titration Experiment With Acid.
From www.vernier.com
AcidBase Titration > Experiment 24 from Chemistry with Vernier Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and. Titration Experiment With Acid.
From stock.adobe.com
Acid base titration experiment and phases of color change during Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. By measuring the temperature change each time a portion of acid is. Determine the concentration of. Titration Experiment With Acid.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titration Experiment With Acid Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. By measuring the temperature change each time a portion. Titration Experiment With Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Experiment With Acid in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. students should be able to: The concentration of the naoh solution is. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. Determine the concentration of analyte present, as well as. Titration Experiment With Acid.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Titration Experiment With Acid By measuring the temperature change each time a portion of acid is. The analyte (titrand) is the solution with an unknown molarity. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in. Titration Experiment With Acid.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Experiment With Acid a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. By measuring the temperature change each time a portion of acid is. The reagent (titrant) is the solution with a known molarity that will react with the analyte. in this experiment, students titrate. Titration Experiment With Acid.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Experiment With Acid in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. students should be able to: in this. Titration Experiment With Acid.
From dxokhzpxh.blob.core.windows.net
Aim Of Acid Base Titration Experiment at Ebony Mooney blog Titration Experiment With Acid The reagent (titrant) is the solution with a known molarity that will react with the analyte. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. The analyte (titrand) is the. Titration Experiment With Acid.
From www.sciencephoto.com
Acidbase titration Stock Image A500/0501 Science Photo Library Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. By measuring the. Titration Experiment With Acid.
From www.flinnsci.com
AcidBase Titrations—Classic Lab Kit for AP® Chemistry Flinn Scientific Titration Experiment With Acid in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. The concentration of the naoh solution is. By measuring the temperature change each time a portion of acid is. students should be able to: a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution. Titration Experiment With Acid.
From mungfali.com
Acid Base Titration Lab Titration Experiment With Acid in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. The concentration of the naoh solution is. a. Titration Experiment With Acid.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Experiment With Acid students should be able to: Describe how to carry out titrations using strong acids and strong alkalis only (sulfuric, hydrochloric and nitric acids only) to find the reacting volumes accurately (ht) calculate the chemical quantities in titrations involving concentrations in mol/dm³ and in g/dm³. a titration is an experiment where a volume of a solution of known concentration. Titration Experiment With Acid.
From mungfali.com
Acid Base Titration Procedure Titration Experiment With Acid in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. By measuring the temperature change each time a portion of acid is. Determine the concentration of analyte present, as well as the acid ionization constant and. students should be able to: The reagent (titrant) is the solution with a known molarity that will react with the analyte.. Titration Experiment With Acid.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Experiment With Acid By measuring the temperature change each time a portion of acid is. Determine the concentration of analyte present, as well as the acid ionization constant and. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The analyte (titrand) is the solution with an. Titration Experiment With Acid.
From mungfali.com
Acid Base Titration Experiment Titration Experiment With Acid Determine the concentration of analyte present, as well as the acid ionization constant and. By measuring the temperature change each time a portion of acid is. The reagent (titrant) is the solution with a known molarity that will react with the analyte. students should be able to: a titration is an experiment where a volume of a solution. Titration Experiment With Acid.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Experiment With Acid The reagent (titrant) is the solution with a known molarity that will react with the analyte. students should be able to: in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. By measuring the temperature change each time a portion of acid is. The analyte (titrand) is the solution with an unknown molarity. Describe how to carry. Titration Experiment With Acid.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Experiment With Acid in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to. The reagent (titrant) is the solution with a known molarity that will react with the analyte.. Titration Experiment With Acid.
From www.youtube.com
Titration Experiment & Calculate the Molarity of Acetic Acid in Vinegar Titration Experiment With Acid The analyte (titrand) is the solution with an unknown molarity. in this experiment, you will titrate hydrochloric acid solution, hcl, with a basic sodium hydroxide solution, naoh. The reagent (titrant) is the solution with a known molarity that will react with the analyte. By measuring the temperature change each time a portion of acid is. Determine the concentration of. Titration Experiment With Acid.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Experiment With Acid students should be able to: The reagent (titrant) is the solution with a known molarity that will react with the analyte. By measuring the temperature change each time a portion of acid is. in this experiment, students titrate sodium hydroxide solution with hydrochloric acid. The concentration of the naoh solution is. Describe how to carry out titrations using. Titration Experiment With Acid.