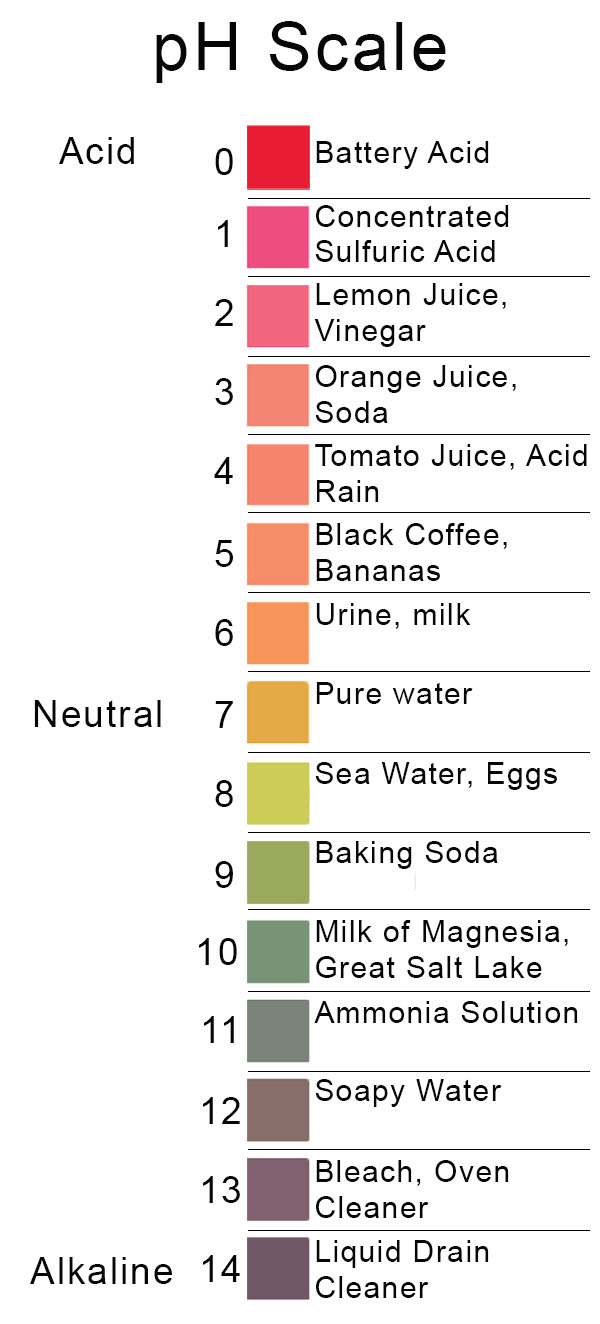
The Limiting Ph Is A Value Below Which Quizlet . the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Ph is usually (but not always) between 0 and 14. the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. define ph and acidic, basic, and neutral ph values. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. Determine the magnitude of change in [h 3 o +] for. The ph scale has no limits. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of.
from www.preclaboratories.com
study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. The ph scale has no limits. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. define ph and acidic, basic, and neutral ph values. the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. Ph is usually (but not always) between 0 and 14. Determine the magnitude of change in [h 3 o +] for.
Back to Basics Acids, Bases & the pH Scale Precision Laboratories
The Limiting Ph Is A Value Below Which Quizlet the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. Determine the magnitude of change in [h 3 o +] for. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. The ph scale has no limits. the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. Ph is usually (but not always) between 0 and 14. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. define ph and acidic, basic, and neutral ph values.
From ideilorjljlessonlearning.z14.web.core.windows.net
Investigating The Ph Scale Answer Key Pdf The Limiting Ph Is A Value Below Which Quizlet Ph is usually (but not always) between 0 and 14. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. define ph and acidic, basic, and neutral ph values. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or. The Limiting Ph Is A Value Below Which Quizlet.
From www.thoughtco.com
pH Definition and Equation in Chemistry The Limiting Ph Is A Value Below Which Quizlet the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. Determine the magnitude of change in [h 3 o +] for. The ph scale has no limits. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. the usual. The Limiting Ph Is A Value Below Which Quizlet.
From d3f6gjnauy613m.cloudfront.net
pHWert einfach erklärt • Skala und Bedeutung · [mit Video] The Limiting Ph Is A Value Below Which Quizlet the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. Determine the magnitude of change in [h 3 o +] for. the usual range of. The Limiting Ph Is A Value Below Which Quizlet.
From jnewbio.edublogs.org
pH levels BioQuakes The Limiting Ph Is A Value Below Which Quizlet the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. Ph is usually (but not always) between 0 and 14. The ph scale has no limits. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. define ph and. The Limiting Ph Is A Value Below Which Quizlet.
From acidsandbasesrios.weebly.com
pH Acids and Bases The Limiting Ph Is A Value Below Which Quizlet study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. Ph is usually (but not always) between 0 and 14. the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. define ph and acidic, basic, and neutral ph values.. The Limiting Ph Is A Value Below Which Quizlet.
From www.pinterest.com
The pH Scale Calculations with pH and pOH Notes and Worksheet Set The Limiting Ph Is A Value Below Which Quizlet Determine the magnitude of change in [h 3 o +] for. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. define ph and acidic, basic, and neutral ph values. the usual range of ph values encountered is between 0 and 14, with 0 being the. The Limiting Ph Is A Value Below Which Quizlet.
From www.albert.io
How to Calculate pH Explanation, Review, and Examples The Limiting Ph Is A Value Below Which Quizlet define ph and acidic, basic, and neutral ph values. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. Ph is usually. The Limiting Ph Is A Value Below Which Quizlet.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG The Limiting Ph Is A Value Below Which Quizlet the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. The ph scale has no limits. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. Ph is usually (but not always) between 0 and 14.. The Limiting Ph Is A Value Below Which Quizlet.
From exonhqfdt.blob.core.windows.net
The Ph Value Quizlet at Sherman Floyd blog The Limiting Ph Is A Value Below Which Quizlet the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. The ph scale has no limits. Ph is usually (but not always) between 0 and 14.. The Limiting Ph Is A Value Below Which Quizlet.
From mrseldred.weebly.com
12 Acids, Bases and Salts Mrs. Eldred's Classroom The Limiting Ph Is A Value Below Which Quizlet The ph scale has no limits. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. Determine the magnitude of change in [h 3 o +]. The Limiting Ph Is A Value Below Which Quizlet.
From www.vecteezy.com
The ph scale diagram 589313 Vector Art at Vecteezy The Limiting Ph Is A Value Below Which Quizlet the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. the ph scale is logarithmic, meaning that an increase or decrease of. The Limiting Ph Is A Value Below Which Quizlet.
From www.expii.com
What Is pH? — Definition & Overview Expii The Limiting Ph Is A Value Below Which Quizlet define ph and acidic, basic, and neutral ph values. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. The ph scale has no limits. Determine the magnitude of change in [h 3 o +] for. the ph scale, which measures from 0 to 14, provides. The Limiting Ph Is A Value Below Which Quizlet.
From www.jagranjosh.com
The Concept and importance of pH Scale The Limiting Ph Is A Value Below Which Quizlet Determine the magnitude of change in [h 3 o +] for. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. The ph scale has no. The Limiting Ph Is A Value Below Which Quizlet.
From izayahferspatterson.blogspot.com
What Is the Ph Value at Which Trypsin Works Best The Limiting Ph Is A Value Below Which Quizlet define ph and acidic, basic, and neutral ph values. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. The ph scale has no limits. . The Limiting Ph Is A Value Below Which Quizlet.
From lessonfullgarbageman.z5.web.core.windows.net
Acids Bases And The Ph Scale The Limiting Ph Is A Value Below Which Quizlet the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. The ph scale has no limits. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. the ph scale, which measures from. The Limiting Ph Is A Value Below Which Quizlet.
From ib.bioninja.com.au
Limiting Factors 2 The Limiting Ph Is A Value Below Which Quizlet study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. the ph scale, which measures from 0 to 14, provides an indication. The Limiting Ph Is A Value Below Which Quizlet.
From www.careerpower.in
pH Values List for Common Substances The Limiting Ph Is A Value Below Which Quizlet The ph scale has no limits. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. Ph is usually (but not always) between 0 and 14. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is.. The Limiting Ph Is A Value Below Which Quizlet.
From www.bbc.co.uk
What is the pH scale and what does it measure? BBC Bitesize The Limiting Ph Is A Value Below Which Quizlet the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. Ph is usually (but not always) between 0 and 14. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. The ph scale has no limits. Determine the magnitude of. The Limiting Ph Is A Value Below Which Quizlet.
From quizlet.com
Find the indicated onesided limit. If the limiting value is Quizlet The Limiting Ph Is A Value Below Which Quizlet Ph is usually (but not always) between 0 and 14. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. the ph scale is logarithmic,. The Limiting Ph Is A Value Below Which Quizlet.
From mavink.com
Ph Scale Diagram The Limiting Ph Is A Value Below Which Quizlet the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. Ph is usually (but not always) between 0 and 14. the ph scale is logarithmic, meaning. The Limiting Ph Is A Value Below Which Quizlet.
From www.chegg.com
Solved For solutions with the following pH values, determine The Limiting Ph Is A Value Below Which Quizlet the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. define ph and acidic, basic, and neutral ph values. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. study with quizlet and memorize. The Limiting Ph Is A Value Below Which Quizlet.
From exonhqfdt.blob.core.windows.net
The Ph Value Quizlet at Sherman Floyd blog The Limiting Ph Is A Value Below Which Quizlet define ph and acidic, basic, and neutral ph values. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. the limiting ph is a. The Limiting Ph Is A Value Below Which Quizlet.
From printabletiroes.z22.web.core.windows.net
Understanding The Ph Scale The Limiting Ph Is A Value Below Which Quizlet the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. Ph is usually (but not always) between 0 and 14. define ph and acidic, basic, and neutral ph values. Determine the magnitude of change in [h 3 o +] for. study with quizlet. The Limiting Ph Is A Value Below Which Quizlet.
From www.preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories The Limiting Ph Is A Value Below Which Quizlet the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated. The Limiting Ph Is A Value Below Which Quizlet.
From www.youtube.com
Limiting Reagent pH calculations YouTube The Limiting Ph Is A Value Below Which Quizlet The ph scale has no limits. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. the limiting ph is a value. The Limiting Ph Is A Value Below Which Quizlet.
From study.com
Acidic, Basic & Neutral Solutions Determining pH Video & Lesson The Limiting Ph Is A Value Below Which Quizlet Ph is usually (but not always) between 0 and 14. The ph scale has no limits. Determine the magnitude of change in [h 3 o +] for. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. the ph scale, which measures from 0 to 14, provides. The Limiting Ph Is A Value Below Which Quizlet.
From sciencenotes.org
How to Calculate pH Formula and Examples The Limiting Ph Is A Value Below Which Quizlet The ph scale has no limits. Ph is usually (but not always) between 0 and 14. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. the ph scale. The Limiting Ph Is A Value Below Which Quizlet.
From www.mometrix.com
pH Overview (Chemistry Review Video) The Limiting Ph Is A Value Below Which Quizlet Ph is usually (but not always) between 0 and 14. The ph scale has no limits. Determine the magnitude of change in [h 3 o +] for. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. define ph and acidic, basic, and neutral ph values. . The Limiting Ph Is A Value Below Which Quizlet.
From mungfali.com
PH Scale Examples The Limiting Ph Is A Value Below Which Quizlet The ph scale has no limits. the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. Ph is usually (but not always) between 0 and 14.. The Limiting Ph Is A Value Below Which Quizlet.
From www.toppr.com
pH Definition, Formula, Meaning, Applications and More The Limiting Ph Is A Value Below Which Quizlet the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. The ph scale has no limits. the usual range of ph values encountered is between. The Limiting Ph Is A Value Below Which Quizlet.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID The Limiting Ph Is A Value Below Which Quizlet Ph is usually (but not always) between 0 and 14. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. the ph scale, which measures. The Limiting Ph Is A Value Below Which Quizlet.
From quizlet.com
limiting factors on photosynthesis Diagram Quizlet The Limiting Ph Is A Value Below Which Quizlet Ph is usually (but not always) between 0 and 14. define ph and acidic, basic, and neutral ph values. study with quizlet and memorize flashcards containing terms like the most important buffer system in the blood is a solution of. Determine the magnitude of change in [h 3 o +] for. the ph scale is logarithmic, meaning. The Limiting Ph Is A Value Below Which Quizlet.
From www.teachoo.com
Strength of Acids and Bases (How to find it?) Chemistry Teachoo The Limiting Ph Is A Value Below Which Quizlet the ph scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. define ph and acidic, basic, and neutral ph values. The ph scale. The Limiting Ph Is A Value Below Which Quizlet.
From byjus.com
pH Of Acids And Bases Calculate pH Value Chemistry Byju's The Limiting Ph Is A Value Below Which Quizlet the limiting ph is a value below which hydrogen ions cannot be secreted into the tubular fluid. The ph scale has no limits. the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. define ph and acidic, basic, and neutral ph values. study with quizlet. The Limiting Ph Is A Value Below Which Quizlet.
From www.ck12.org
Water, Acids, and Bases CK12 Foundation The Limiting Ph Is A Value Below Which Quizlet the ph scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. the usual range of ph values encountered is between 0 and 14, with 0 being the value for concentrated hydrochloric acid (1 m hcl),. The ph scale has no limits. study with quizlet and memorize flashcards. The Limiting Ph Is A Value Below Which Quizlet.