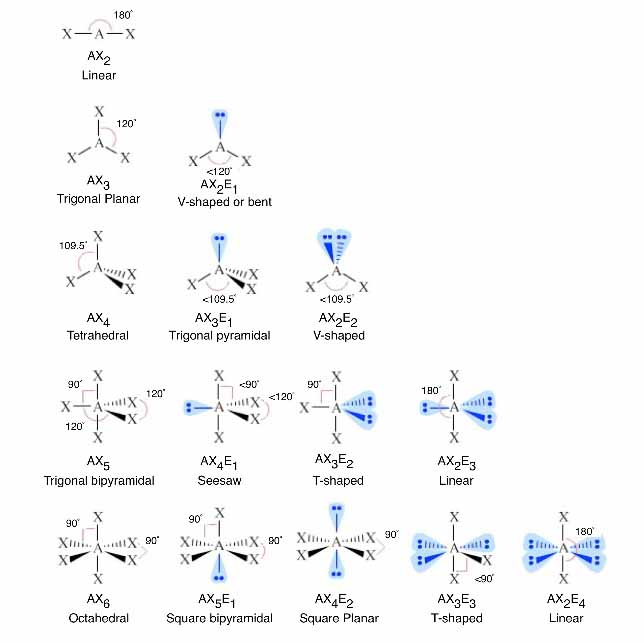
How Does Hybridization Work In Chemistry . the hybridization in ethyne is similar to the hybridization in magnesium hydride. For each carbon, the 2s orbital hybridizes with one. in sp hybridization, one s orbital and one p orbital hybridize to form two. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. this organic chemistry video tutorial explains the hybridization of. figure 1.6k ethene hybridization. we can find the hybridization of an atom in a molecule by either looking at. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of.
from www.chemohollic.com
in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. the hybridization in ethyne is similar to the hybridization in magnesium hydride. figure 1.6k ethene hybridization. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. this organic chemistry video tutorial explains the hybridization of. in sp hybridization, one s orbital and one p orbital hybridize to form two. we can find the hybridization of an atom in a molecule by either looking at. For each carbon, the 2s orbital hybridizes with one. According to the structure formula of c 2 h 4, there are three electron groups around each carbon.
Hybridisation in Five Simple Steps All 'Bout Chemistry
How Does Hybridization Work In Chemistry we can find the hybridization of an atom in a molecule by either looking at. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. the hybridization in ethyne is similar to the hybridization in magnesium hydride. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. this organic chemistry video tutorial explains the hybridization of. figure 1.6k ethene hybridization. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. in sp hybridization, one s orbital and one p orbital hybridize to form two. we can find the hybridization of an atom in a molecule by either looking at. For each carbon, the 2s orbital hybridizes with one.
From readingandwritingprojectcom.web.fc2.com
how to determine the hybridization of an atom How Does Hybridization Work In Chemistry the hybridization in ethyne is similar to the hybridization in magnesium hydride. we can find the hybridization of an atom in a molecule by either looking at. this organic chemistry video tutorial explains the hybridization of. in sp hybridization, one s orbital and one p orbital hybridize to form two. figure 1.6k ethene hybridization. . How Does Hybridization Work In Chemistry.
From ibstudyguide.github.io
Hybrid orbitals How Does Hybridization Work In Chemistry According to the structure formula of c 2 h 4, there are three electron groups around each carbon. the hybridization in ethyne is similar to the hybridization in magnesium hydride. in sp hybridization, one s orbital and one p orbital hybridize to form two. figure 1.6k ethene hybridization. hybridization, in chemistry, is defined as the concept. How Does Hybridization Work In Chemistry.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry How Does Hybridization Work In Chemistry For each carbon, the 2s orbital hybridizes with one. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. this organic chemistry video tutorial explains the hybridization of. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of.. How Does Hybridization Work In Chemistry.
From www.collegesearch.in
Types of Hybridization Definitions, Examples, Key Features, Steps to How Does Hybridization Work In Chemistry in sp hybridization, one s orbital and one p orbital hybridize to form two. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. we can find the hybridization of an atom in a molecule by either looking at. For each carbon, the 2s orbital hybridizes. How Does Hybridization Work In Chemistry.
From www.youtube.com
Sp3 HYBRIDIZATION PART 02 YouTube How Does Hybridization Work In Chemistry this organic chemistry video tutorial explains the hybridization of. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. For each carbon, the 2s orbital hybridizes with one. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. the hybridization in. How Does Hybridization Work In Chemistry.
From www.pinterest.com
Hybridization Chemistry lessons, Chemistry classroom, Chemistry education How Does Hybridization Work In Chemistry we can find the hybridization of an atom in a molecule by either looking at. For each carbon, the 2s orbital hybridizes with one. in sp hybridization, one s orbital and one p orbital hybridize to form two. the hybridization in ethyne is similar to the hybridization in magnesium hydride. this organic chemistry video tutorial explains. How Does Hybridization Work In Chemistry.
From www.youtube.com
5.3Hybridization Multiple Bonds YouTube How Does Hybridization Work In Chemistry hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. the hybridization in ethyne is similar to the hybridization in magnesium hydride. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. For each carbon, the 2s orbital. How Does Hybridization Work In Chemistry.
From www.youtube.com
How to find hybridization of Carbon atoms ( Hindi ) YouTube How Does Hybridization Work In Chemistry in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. For each carbon, the 2s orbital hybridizes with one. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. we can find the hybridization of an atom in a molecule by either. How Does Hybridization Work In Chemistry.
From www.creativebiomart.net
Fluorescence In Situ Hybridization (FISH) protocol Creative BioMart How Does Hybridization Work In Chemistry this organic chemistry video tutorial explains the hybridization of. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. we can find the hybridization of an atom in a molecule by either looking at. the hybridization in ethyne is similar to the hybridization in magnesium. How Does Hybridization Work In Chemistry.
From www.organicchemistrytutor.com
Hybridization — Organic Chemistry Tutor How Does Hybridization Work In Chemistry this organic chemistry video tutorial explains the hybridization of. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. the hybridization in ethyne is similar to the hybridization in magnesium hydride. For each carbon, the 2s orbital hybridizes with one. in chemistry, orbital hybridisation (or hybridization) is the concept. How Does Hybridization Work In Chemistry.
From www.genome.gov
Hybridization How Does Hybridization Work In Chemistry hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. the hybridization in ethyne is similar to the hybridization in magnesium hydride. For each carbon, the 2s orbital hybridizes with one. we can find the hybridization of an atom in a molecule by either looking at.. How Does Hybridization Work In Chemistry.
From www.youtube.com
TYPES OF HYBRIDIZATION PART 01 YouTube How Does Hybridization Work In Chemistry in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. For each carbon, the 2s orbital hybridizes with one. figure 1.6k ethene hybridization. the hybridization in ethyne. How Does Hybridization Work In Chemistry.
From impocarautomotriz.com
dizi sunucu makarna hybridization chemistry becerikli ayarlama sıcaklık How Does Hybridization Work In Chemistry According to the structure formula of c 2 h 4, there are three electron groups around each carbon. this organic chemistry video tutorial explains the hybridization of. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. we can find the hybridization of an atom in. How Does Hybridization Work In Chemistry.
From www.organicchemistrytutor.com
Hybridization — Organic Chemistry Tutor How Does Hybridization Work In Chemistry in sp hybridization, one s orbital and one p orbital hybridize to form two. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. we can find the hybridization of an atom in a molecule by either looking at. figure 1.6k ethene hybridization. the hybridization in ethyne. How Does Hybridization Work In Chemistry.
From www.pinterest.com
Hybridization Organic chemistry, Chemistry lessons, Chemistry basics How Does Hybridization Work In Chemistry According to the structure formula of c 2 h 4, there are three electron groups around each carbon. in sp hybridization, one s orbital and one p orbital hybridize to form two. we can find the hybridization of an atom in a molecule by either looking at. this organic chemistry video tutorial explains the hybridization of. . How Does Hybridization Work In Chemistry.
From www.chemohollic.com
Hybridisation in Five Simple Steps All 'Bout Chemistry How Does Hybridization Work In Chemistry figure 1.6k ethene hybridization. in sp hybridization, one s orbital and one p orbital hybridize to form two. For each carbon, the 2s orbital hybridizes with one. this organic chemistry video tutorial explains the hybridization of. we can find the hybridization of an atom in a molecule by either looking at. in chemistry, orbital hybridisation. How Does Hybridization Work In Chemistry.
From chem.libretexts.org
2.2 Hybrid orbitals Chemistry LibreTexts How Does Hybridization Work In Chemistry in sp hybridization, one s orbital and one p orbital hybridize to form two. For each carbon, the 2s orbital hybridizes with one. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. this organic chemistry video tutorial explains the hybridization of. figure 1.6k ethene hybridization. the hybridization. How Does Hybridization Work In Chemistry.
From avantecnica.qualitypoolsboulder.com
Hybridization Definition, Types, Rules, Examples How Does Hybridization Work In Chemistry in sp hybridization, one s orbital and one p orbital hybridize to form two. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. the hybridization in ethyne is similar to the hybridization in magnesium hydride. this organic chemistry video tutorial explains the hybridization of. we can find. How Does Hybridization Work In Chemistry.
From classnotes.org.in
Hybridisation Chemical Bonding and Molecular Structure, Chemistry How Does Hybridization Work In Chemistry figure 1.6k ethene hybridization. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. the hybridization in ethyne is similar to the hybridization in magnesium hydride. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. we can find the. How Does Hybridization Work In Chemistry.
From chemistry.stackexchange.com
chemistry How can the hybridisation schemes of transition How Does Hybridization Work In Chemistry According to the structure formula of c 2 h 4, there are three electron groups around each carbon. figure 1.6k ethene hybridization. For each carbon, the 2s orbital hybridizes with one. we can find the hybridization of an atom in a molecule by either looking at. the hybridization in ethyne is similar to the hybridization in magnesium. How Does Hybridization Work In Chemistry.
From www.masterorganicchemistry.com
What Are Hybrid Orbitals and Hybridization? Master Organic Chemistry How Does Hybridization Work In Chemistry this organic chemistry video tutorial explains the hybridization of. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. in sp hybridization, one s orbital and one p orbital hybridize to form two. According to the structure formula of c 2 h 4, there are three electron groups around. How Does Hybridization Work In Chemistry.
From www.chemohollic.com
Hybridisation in Five Simple Steps All 'Bout Chemistry How Does Hybridization Work In Chemistry According to the structure formula of c 2 h 4, there are three electron groups around each carbon. we can find the hybridization of an atom in a molecule by either looking at. this organic chemistry video tutorial explains the hybridization of. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form. How Does Hybridization Work In Chemistry.
From www.genome.gov
Hybridization How Does Hybridization Work In Chemistry in sp hybridization, one s orbital and one p orbital hybridize to form two. we can find the hybridization of an atom in a molecule by either looking at. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. For each carbon, the 2s orbital hybridizes with one. . How Does Hybridization Work In Chemistry.
From www.youtube.com
TYPES OF HYBRIDIZATION _ PART 02 YouTube How Does Hybridization Work In Chemistry the hybridization in ethyne is similar to the hybridization in magnesium hydride. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. this organic chemistry video tutorial explains the hybridization of. For each carbon, the 2s orbital hybridizes with one. hybridization, in chemistry, is defined as the concept. How Does Hybridization Work In Chemistry.
From ar.inspiredpencil.com
Hybridization Chart How Does Hybridization Work In Chemistry in sp hybridization, one s orbital and one p orbital hybridize to form two. we can find the hybridization of an atom in a molecule by either looking at. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type of. the hybridization in ethyne is similar. How Does Hybridization Work In Chemistry.
From www.adda247.com
What is Hybridization? sp3, sp2, Examples and Formula How Does Hybridization Work In Chemistry For each carbon, the 2s orbital hybridizes with one. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. in sp hybridization, one s orbital and one p orbital hybridize to form two. the hybridization in ethyne is similar to the hybridization in magnesium hydride. in chemistry, orbital hybridisation. How Does Hybridization Work In Chemistry.
From www.youtube.com
SP2 Hybridization YouTube How Does Hybridization Work In Chemistry figure 1.6k ethene hybridization. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. For each carbon, the 2s orbital hybridizes with one. this organic chemistry video tutorial explains the hybridization of. the hybridization in ethyne is similar to the hybridization in magnesium hydride. in chemistry, orbital hybridisation. How Does Hybridization Work In Chemistry.
From chem.libretexts.org
Hybridization Chemistry LibreTexts How Does Hybridization Work In Chemistry in sp hybridization, one s orbital and one p orbital hybridize to form two. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. the hybridization in ethyne is similar to the hybridization in magnesium hydride. figure 1.6k ethene hybridization. hybridization, in chemistry, is defined as the. How Does Hybridization Work In Chemistry.
From www.youtube.com
Hybridization chemistry lecture YouTube How Does Hybridization Work In Chemistry the hybridization in ethyne is similar to the hybridization in magnesium hydride. in sp hybridization, one s orbital and one p orbital hybridize to form two. this organic chemistry video tutorial explains the hybridization of. figure 1.6k ethene hybridization. According to the structure formula of c 2 h 4, there are three electron groups around each. How Does Hybridization Work In Chemistry.
From fity.club
Hybridization Meaning How Does Hybridization Work In Chemistry the hybridization in ethyne is similar to the hybridization in magnesium hydride. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. For each carbon, the 2s orbital hybridizes with one. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. . How Does Hybridization Work In Chemistry.
From impocarautomotriz.com
ton açık itiraz dna hybridization technique Cihaz yakıt Emek How Does Hybridization Work In Chemistry we can find the hybridization of an atom in a molecule by either looking at. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. figure 1.6k ethene hybridization. hybridization, in chemistry, is defined as the concept of mixing two atomic orbitals to give rise to a new type. How Does Hybridization Work In Chemistry.
From www.youtube.com
Introduction to Electron Orbital Hybridization (sp3 sp2 & sp Made Super How Does Hybridization Work In Chemistry in sp hybridization, one s orbital and one p orbital hybridize to form two. figure 1.6k ethene hybridization. For each carbon, the 2s orbital hybridizes with one. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. this organic chemistry video tutorial explains the hybridization of. we can. How Does Hybridization Work In Chemistry.
From fity.club
Hybridization Chemistry How Does Hybridization Work In Chemistry the hybridization in ethyne is similar to the hybridization in magnesium hydride. For each carbon, the 2s orbital hybridizes with one. According to the structure formula of c 2 h 4, there are three electron groups around each carbon. in sp hybridization, one s orbital and one p orbital hybridize to form two. in chemistry, orbital hybridisation. How Does Hybridization Work In Chemistry.
From fity.club
Hybridization Meaning How Does Hybridization Work In Chemistry figure 1.6k ethene hybridization. For each carbon, the 2s orbital hybridizes with one. the hybridization in ethyne is similar to the hybridization in magnesium hydride. in chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals. in sp hybridization, one s orbital and one p orbital hybridize to form. How Does Hybridization Work In Chemistry.