Electron Configuration Formula Of Tin . Full electron configuration of tin: The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. And thus 50 electrons must be. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Indium ← tin → antimony. Second, make a table of subshell and its maximum electrons. Where, ℓ = azimuthal quantum number of the subshell Since the atomic number of tin is 50, the total electrons of tin are 50. The atomic number of tin represents the total number of electrons of tin. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. Key concepts these are the key. Calculate the maximum number of electrons each subshell can hold using the formula: Electron configuration chart of all elements is mentioned in the table below.
from general.chemistrysteps.com
The shorthand electron configuration (or noble gas configuration) as well as. Calculate the maximum number of electrons each subshell can hold using the formula: Electron configuration chart of all elements is mentioned in the table below. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Where, ℓ = azimuthal quantum number of the subshell Full electron configuration of tin: Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). And thus 50 electrons must be. Since the atomic number of tin is 50, the total electrons of tin are 50.
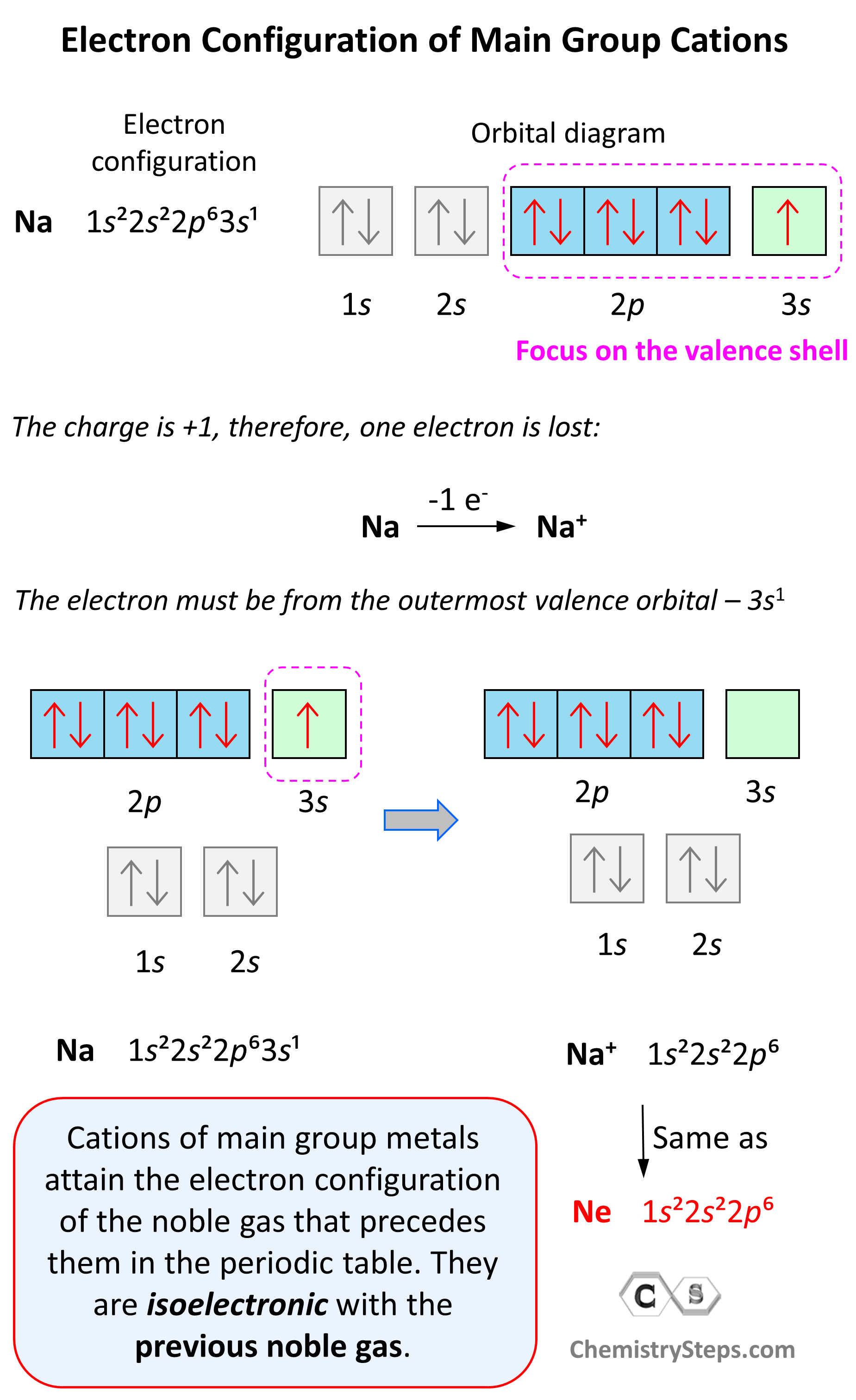
Electron Configurations of Ions Chemistry Steps
Electron Configuration Formula Of Tin Electron configuration chart of all elements is mentioned in the table below. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Electron configuration chart of all elements is mentioned in the table below. Full electron configuration of tin: Calculate the maximum number of electrons each subshell can hold using the formula: Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. Second, make a table of subshell and its maximum electrons. Indium ← tin → antimony. The shorthand electron configuration (or noble gas configuration) as well as. Since the atomic number of tin is 50, the total electrons of tin are 50. The atomic number of tin represents the total number of electrons of tin. Where, ℓ = azimuthal quantum number of the subshell And thus 50 electrons must be. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Key concepts these are the key.
From lessoncampusunspelt.z13.web.core.windows.net
Electron Configuration And Orbital Notation Worksheet 2 Answ Electron Configuration Formula Of Tin Indium ← tin → antimony. And thus 50 electrons must be. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Second, make a table of subshell and its maximum. Electron Configuration Formula Of Tin.
From www.numerade.com
SOLVED(a) What is the electron configuration for an atom of tin? (b Electron Configuration Formula Of Tin The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Key concepts these are the key. Full electron configuration of tin: Indium ← tin → antimony. The atomic number of tin represents the total number of electrons of tin. And thus 50 electrons must be. 1s2 2s2 2p6 3s2. Electron Configuration Formula Of Tin.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Electron Configuration Formula Of Tin The shorthand electron configuration (or noble gas configuration) as well as. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. Second, make a table of subshell and its maximum. Electron Configuration Formula Of Tin.
From www.numerade.com
SOLVED Write the electron configuration of the following elements or Electron Configuration Formula Of Tin Indium ← tin → antimony. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. The shorthand electron configuration (or noble. Electron Configuration Formula Of Tin.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Electron Configuration Formula Of Tin Electron configuration chart of all elements is mentioned in the table below. The atomic number of tin represents the total number of electrons of tin. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5. Electron Configuration Formula Of Tin.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Electron Configuration Formula Of Tin The atomic number of tin represents the total number of electrons of tin. Key concepts these are the key. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. And thus 50 electrons must be. The shorthand electron configuration (or noble gas configuration) as well as. Since the atomic number of tin is 50, the total electrons of tin. Electron Configuration Formula Of Tin.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Electron Configuration Formula Of Tin The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Second, make a table of subshell and its maximum electrons. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d. Electron Configuration Formula Of Tin.
From www.youtube.com
Sn^2+,Sn^4+ and Sn (Tin and Tin Ions) Electron ConfigurationCrash Electron Configuration Formula Of Tin Key concepts these are the key. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Full electron configuration of tin: Where, ℓ = azimuthal quantum number of the subshell The atomic number of tin represents the total number of electrons of tin. The shorthand electron configuration (or noble gas configuration) as well. Electron Configuration Formula Of Tin.
From ar.inspiredpencil.com
Electron Configuration Electron Configuration Formula Of Tin Electron configuration chart of all elements is mentioned in the table below. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. And thus 50 electrons must be. Full electron configuration of tin: Key concepts these are the key. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3. Electron Configuration Formula Of Tin.
From valenceelectrons.com
What is Electron Configuration and Why it’s Important? Electron Configuration Formula Of Tin The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). The atomic number of tin represents the total number of electrons of tin. Calculate the maximum number of electrons each subshell can hold using the formula: Key concepts these are the key. Second, make a table of subshell and its maximum electrons. Full. Electron Configuration Formula Of Tin.
From www.scribd.com
Electron Configuration Formula PDF Electron Configuration Formula Of Tin Key concepts these are the key. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. The shorthand electron configuration (or noble gas configuration) as well as. Where, ℓ =. Electron Configuration Formula Of Tin.
From keplarllp.com
😍 Electron configuration examples. Abbreviated Electron configurations Electron Configuration Formula Of Tin Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. Electron configuration chart of all elements is mentioned in the table below. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6. Electron Configuration Formula Of Tin.
From www.slideshare.net
Electron configuration Electron Configuration Formula Of Tin Second, make a table of subshell and its maximum electrons. Electron configuration chart of all elements is mentioned in the table below. Key concepts these are the key. Indium ← tin → antimony. The shorthand electron configuration (or noble gas configuration) as well as. The atomic number of tin represents the total number of electrons of tin. Since the atomic. Electron Configuration Formula Of Tin.
From www.youtube.com
How to Find the Valence Electrons for Tin (Sn) YouTube Electron Configuration Formula Of Tin Key concepts these are the key. Full electron configuration of tin: And thus 50 electrons must be. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Electron configuration chart of all elements is mentioned in the table below. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3. Electron Configuration Formula Of Tin.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Electron Configuration Formula Of Tin Since the atomic number of tin is 50, the total electrons of tin are 50. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Indium ← tin → antimony. Calculate the maximum number of electrons each subshell can hold using the formula: Second, make a table of subshell and its maximum electrons. Where, ℓ = azimuthal quantum number. Electron Configuration Formula Of Tin.
From www.chemistrylearner.com
Tin Definition, Facts, Symbol, Discovery, Property, Uses Electron Configuration Formula Of Tin Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. Full electron configuration of tin: And thus 50 electrons must be. Since the atomic number of tin is 50, the. Electron Configuration Formula Of Tin.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Electron Configuration Formula Of Tin Full electron configuration of tin: The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Electron configuration chart of all elements is mentioned in the table below. Calculate the maximum number of electrons each subshell can hold using the formula: And thus 50 electrons must be. Second, make a. Electron Configuration Formula Of Tin.
From www.colourbox.com
Symbol and electron diagram for Tin illustration Stock Vector Colourbox Electron Configuration Formula Of Tin Indium ← tin → antimony. Where, ℓ = azimuthal quantum number of the subshell Key concepts these are the key. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\).. Electron Configuration Formula Of Tin.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration Electron Configuration Formula Of Tin Indium ← tin → antimony. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. The atomic number of tin represents the total number of electrons of tin. The shorthand. Electron Configuration Formula Of Tin.
From www.thoughtco.com
Electron Configuration Chart Electron Configuration Formula Of Tin The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). And thus 50 electrons must be. Where, ℓ = azimuthal. Electron Configuration Formula Of Tin.
From ar.inspiredpencil.com
Tin Atomic Structure Electron Configuration Formula Of Tin Indium ← tin → antimony. Where, ℓ = azimuthal quantum number of the subshell The atomic number of tin represents the total number of electrons of tin. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Key concepts these are the key. The shorthand electron configuration (or noble gas configuration) as well. Electron Configuration Formula Of Tin.
From www.alamy.com
Sn Tin, Periodic Table of the Elements, Shell Structure of Tin Electron Configuration Formula Of Tin Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 10 4 s 2 4 p 6 4 d 10 5 s 2 5 p 2. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of tin is \(1s^2, 2s^2, 2p^6,. Electron Configuration Formula Of Tin.
From valenceelectrons.com
Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+) Electron Configuration Formula Of Tin Second, make a table of subshell and its maximum electrons. Since the atomic number of tin is 50, the total electrons of tin are 50. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. The atomic number of tin represents the total number of electrons of tin. Electron configuration chart of all elements is mentioned in the table. Electron Configuration Formula Of Tin.
From aurorecollette.blogspot.com
orbital diagram of tin AuroreCollette Electron Configuration Formula Of Tin The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Electron configuration chart of all elements is mentioned in the table below. Calculate the maximum number of electrons each subshell can hold using the formula: The shorthand electron configuration (or noble gas configuration) as well as. And thus 50. Electron Configuration Formula Of Tin.
From www.youtube.com
Electron Configuration of Tin Sn Lesson YouTube Electron Configuration Formula Of Tin The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). The shorthand electron configuration (or noble gas configuration) as well as. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3 s 2 3 p. Electron Configuration Formula Of Tin.
From sciencenotes.org
List of Electron Configurations of Elements Electron Configuration Formula Of Tin The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Where, ℓ = azimuthal quantum number of the subshell Since the atomic number of tin is 50, the total electrons of tin are 50. Therefore, the electronic configuration of tin is 1 s 2 2 s 2. Electron Configuration Formula Of Tin.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Electron Configuration Formula Of Tin Second, make a table of subshell and its maximum electrons. Indium ← tin → antimony. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Where, ℓ = azimuthal quantum number of the subshell 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. The atomic number of tin represents the total. Electron Configuration Formula Of Tin.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Electron Configuration Formula Of Tin The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Since the atomic number of tin is 50, the total electrons of tin are 50. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Where, ℓ = azimuthal quantum number of the subshell Therefore, the electronic configuration of tin is 1. Electron Configuration Formula Of Tin.
From www.youtube.com
Full and Abbreviated Electron Configuration of Ruthenium Ru YouTube Electron Configuration Formula Of Tin Calculate the maximum number of electrons each subshell can hold using the formula: Since the atomic number of tin is 50, the total electrons of tin are 50. The atomic number of tin represents the total number of electrons of tin. Second, make a table of subshell and its maximum electrons. Therefore, the electronic configuration of tin is 1 s. Electron Configuration Formula Of Tin.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Electron Configuration Formula Of Tin 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Indium ← tin → antimony. Electron configuration chart of all elements is mentioned in the table below. Since the atomic number of tin is 50, the total electrons of tin are 50. Therefore, the electronic configuration of tin is 1 s 2 2 s 2 2 p 6 3. Electron Configuration Formula Of Tin.
From animalia-life.club
Electron Configuration Of Arsenic Electron Configuration Formula Of Tin Since the atomic number of tin is 50, the total electrons of tin are 50. Where, ℓ = azimuthal quantum number of the subshell The shorthand electron configuration (or noble gas configuration) as well as. Second, make a table of subshell and its maximum electrons. The tin orbital diagram is a graphical representation of the electron configuration of the element. Electron Configuration Formula Of Tin.
From holooly.com
Use the periodic table to write the shorthand electron configuration of Electron Configuration Formula Of Tin Indium ← tin → antimony. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Calculate the maximum number of electrons each subshell can hold using the formula: Since the atomic number of tin is 50, the total electrons of tin. Electron Configuration Formula Of Tin.
From valenceelectrons.com
Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+) Electron Configuration Formula Of Tin Full electron configuration of tin: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Second, make a table of subshell and its maximum electrons. Where, ℓ = azimuthal quantum number of the subshell Electron configuration chart of all elements is mentioned in the table below. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10},. Electron Configuration Formula Of Tin.
From classnotes.org.in
Physical Properties Of Carbon Family Chemistry, Class 11, pBlock Electron Configuration Formula Of Tin Electron configuration chart of all elements is mentioned in the table below. Full electron configuration of tin: The atomic number of tin represents the total number of electrons of tin. Since the atomic number of tin is 50, the total electrons of tin are 50. Indium ← tin → antimony. Where, ℓ = azimuthal quantum number of the subshell The. Electron Configuration Formula Of Tin.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Electron Configuration Formula Of Tin Electron configuration chart of all elements is mentioned in the table below. Second, make a table of subshell and its maximum electrons. The electron configuration of tin is \(1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^{10}, 4p^6, 5s^2, 4d^{10}, 5p^2\). Since the atomic number of tin is 50, the total electrons of tin are 50. And thus 50 electrons must be.. Electron Configuration Formula Of Tin.