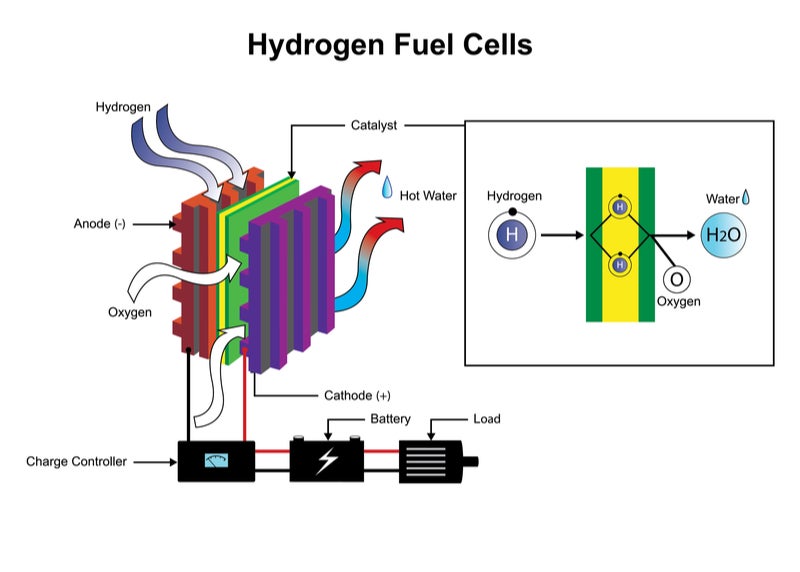
Fuel Cell Hydrogen Consumption Calculation . The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The fuel efficiency, defined as the ratio. A compressor and humidifier), the fuel gas supply, thermal. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The (chemical) energy content of any fuel is called heating value (for. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The use of hhv here is.
from www.naturespeakz.com
The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. The (chemical) energy content of any fuel is called heating value (for. The use of hhv here is. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. A compressor and humidifier), the fuel gas supply, thermal. The fuel efficiency, defined as the ratio.
Why Hydrogen Is The Fuel Of The Future Nature Speakz
Fuel Cell Hydrogen Consumption Calculation The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The fuel efficiency, defined as the ratio. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The use of hhv here is. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. The (chemical) energy content of any fuel is called heating value (for. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. A compressor and humidifier), the fuel gas supply, thermal.
From www.vecteezy.com
Hydrogen oxygen fuel cell 21669326 Vector Art at Vecteezy Fuel Cell Hydrogen Consumption Calculation Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The fuel efficiency, defined as the ratio. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The use of hhv here is. The (chemical) energy content of any fuel is called. Fuel Cell Hydrogen Consumption Calculation.
From www.studypool.com
SOLUTION Hydrogen fuel cell Studypool Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. The use of hhv here is. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%).. Fuel Cell Hydrogen Consumption Calculation.
From www.globenewswire.com
Hydrogen Fuel Cells Market Size to Surpass US 131.06 Bn by Fuel Cell Hydrogen Consumption Calculation The use of hhv here is. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. The (chemical) energy content of any fuel is called heating value (for. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The fuel efficiency, defined as the ratio. A compressor and humidifier), the fuel. Fuel Cell Hydrogen Consumption Calculation.
From www.researchgate.net
Operating costs of hydrogen fuel cell vehicles as a function of Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. The use of hhv here is. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The (chemical) energy content of any fuel is called heating value (for. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy. Fuel Cell Hydrogen Consumption Calculation.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cell Hydrogen Consumption Calculation The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The power produced by a fuel cell depends on several factors, including the. Fuel Cell Hydrogen Consumption Calculation.
From www.simscale.com
Hydrogen Fuel Cell Simulation & Modeling Blog SimScale Fuel Cell Hydrogen Consumption Calculation The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The fuel efficiency, defined as the ratio. The (chemical) energy content of any fuel is called heating value (for. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2. Fuel Cell Hydrogen Consumption Calculation.
From www.mdpi.com
Applied Sciences Free FullText Portable Prototype of Hydrogen Fuel Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. A compressor and humidifier), the fuel gas supply, thermal. The most efficient method is to compress the hydrogen. Fuel Cell Hydrogen Consumption Calculation.
From ecity.solarisbus.com
Hydrogen as the fuel of the future? eCity powered by Solaris Fuel Cell Hydrogen Consumption Calculation Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The power produced by a fuel cell depends on several factors,. Fuel Cell Hydrogen Consumption Calculation.
From www.freepik.com
Premium Vector Green hydrogen fuel cell h2 energy plant clean power Fuel Cell Hydrogen Consumption Calculation The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The (chemical) energy content of any fuel is called heating value (for. The paper discusses the hydrogen consumption calculation of proton exchange. Fuel Cell Hydrogen Consumption Calculation.
From www.numerade.com
SOLVED The efficiency of a fuel cell is related to the average voltage Fuel Cell Hydrogen Consumption Calculation The use of hhv here is. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The (chemical). Fuel Cell Hydrogen Consumption Calculation.
From www.enr.com
Global Upswing Forecast in HydrogenFueled Energy Production 202206 Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. In the case of the hydrogen fuel cell this value is the gibbs. Fuel Cell Hydrogen Consumption Calculation.
From moeentatyana.blogspot.com
Mileage and petrol calculator MoeenTatyana Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. A compressor and humidifier), the fuel gas supply, thermal. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The (chemical) energy content of any. Fuel Cell Hydrogen Consumption Calculation.
From www.animalia-life.club
Toyota Hydrogen Fuel Cell Diagram Fuel Cell Hydrogen Consumption Calculation The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The use of hhv here is. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. The. Fuel Cell Hydrogen Consumption Calculation.
From www.animalia-life.club
Toyota Hydrogen Fuel Cell Diagram Fuel Cell Hydrogen Consumption Calculation A compressor and humidifier), the fuel gas supply, thermal. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios. Fuel Cell Hydrogen Consumption Calculation.
From totalshield.com
Electrolyzer and Hydrogen Fuel Cell Safety TotalShield Fuel Cell Hydrogen Consumption Calculation The (chemical) energy content of any fuel is called heating value (for. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The fuel efficiency, defined as the ratio. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. In. Fuel Cell Hydrogen Consumption Calculation.
From driveelectric.org.nz
Hydrogen Fuel Cell Electric Vehicles Drive Electric Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The (chemical) energy content of any fuel is called heating value (for. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The use of hhv. Fuel Cell Hydrogen Consumption Calculation.
From www.researchgate.net
Fuel cell system structure Hydrogen and air are compressed and Fuel Cell Hydrogen Consumption Calculation The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. A compressor and humidifier), the fuel gas supply, thermal. In the case of the hydrogen fuel cell this value is the gibbs free. Fuel Cell Hydrogen Consumption Calculation.
From encyclopedia.pub
HydrogenBased Hybrid Microgrid System Encyclopedia MDPI Fuel Cell Hydrogen Consumption Calculation The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The paper discusses the hydrogen consumption calculation of proton exchange membrane. Fuel Cell Hydrogen Consumption Calculation.
From www.researchgate.net
Fuel cell hydrogen consumption vs. power production. Download Fuel Cell Hydrogen Consumption Calculation The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. A compressor and humidifier), the fuel gas supply, thermal. The fuel efficiency, defined as the ratio. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8. Fuel Cell Hydrogen Consumption Calculation.
From futureentech.com
Information Trends Estimates 20 Million Sales and 1.2 Trillion Revenue Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The use of hhv here is. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The (chemical) energy content of any fuel is called. Fuel Cell Hydrogen Consumption Calculation.
From ar.inspiredpencil.com
Auto Hydrogen Fuel Cells Fuel Cell Hydrogen Consumption Calculation The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The use of hhv here is. Typically, the reformate fuel cells. Fuel Cell Hydrogen Consumption Calculation.
From www.researchgate.net
Fuel cell system efficiency and hydrogen consumption rate. Download Fuel Cell Hydrogen Consumption Calculation The (chemical) energy content of any fuel is called heating value (for. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The use of hhv here is. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The most efficient method is to compress. Fuel Cell Hydrogen Consumption Calculation.
From www.precedenceresearch.com
Hydrogen Fuel Cells Market Size, Growth, Trends, Report 20232032 Fuel Cell Hydrogen Consumption Calculation The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. A compressor and humidifier), the fuel gas supply, thermal. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The most efficient method is to compress the hydrogen to 680 atm but that. Fuel Cell Hydrogen Consumption Calculation.
From www.vecteezy.com
Green hydrogen energy fuel cell diagram layout system h2 to electric Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The use of hhv here is. The most efficient method is to compress the hydrogen to 680 atm. Fuel Cell Hydrogen Consumption Calculation.
From www.mdpi.com
WEVJ Free FullText Challenges and Solutions of Hydrogen Fuel Cells Fuel Cell Hydrogen Consumption Calculation The use of hhv here is. A compressor and humidifier), the fuel gas supply, thermal. The (chemical) energy content of any fuel is called heating value (for. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The fuel efficiency, defined as the ratio. The power produced by. Fuel Cell Hydrogen Consumption Calculation.
From wha-international.com
Top Industrial Uses of Hydrogen Industrial Hydrogen Safety Fuel Cell Hydrogen Consumption Calculation The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The (chemical) energy content of any fuel is called heating value (for. A compressor and humidifier), the fuel gas supply, thermal. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The use. Fuel Cell Hydrogen Consumption Calculation.
From mavink.com
Hydrogen Fuel Cell Design Fuel Cell Hydrogen Consumption Calculation The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. A compressor and humidifier), the fuel gas supply, thermal. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The (chemical) energy content of any fuel is called heating value (for. The. Fuel Cell Hydrogen Consumption Calculation.
From www.vectorstock.com
Hydrogen fuel cell technology concept Royalty Free Vector Fuel Cell Hydrogen Consumption Calculation The (chemical) energy content of any fuel is called heating value (for. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The reaction enthalpy ∆rh is equal to the enthalpy of water formation ∆fh. The power produced by a fuel cell depends on several. Fuel Cell Hydrogen Consumption Calculation.
From www.naturespeakz.com
Why Hydrogen Is The Fuel Of The Future Nature Speakz Fuel Cell Hydrogen Consumption Calculation The fuel efficiency, defined as the ratio. The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The paper discusses the hydrogen consumption. Fuel Cell Hydrogen Consumption Calculation.
From www.researchgate.net
Schematic diagram of hydrogen fuel cell Download Scientific Diagram Fuel Cell Hydrogen Consumption Calculation The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. A compressor and humidifier), the fuel gas supply, thermal. The use of hhv here is. The fuel efficiency, defined as the ratio. In the case of the hydrogen fuel cell. Fuel Cell Hydrogen Consumption Calculation.
From powertorque.com.au
Cummins Hydrogen FUEL CELL infographic_ Power Torque Fuel Cell Hydrogen Consumption Calculation In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The most efficient method is to compress the hydrogen to 680 atm but that requires about 13% of the total energy content of the hydrogen. The power produced by a fuel cell depends on several factors, including the. Fuel Cell Hydrogen Consumption Calculation.
From cepconsult.com
The development of hydrogen energy CEPCONSULT Fuel Cell Hydrogen Consumption Calculation Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The most efficient method is to. Fuel Cell Hydrogen Consumption Calculation.
From www.designlife-cycle.com
Hydrogen Fuel Cell — Design LifeCycle Fuel Cell Hydrogen Consumption Calculation A compressor and humidifier), the fuel gas supply, thermal. The fuel efficiency, defined as the ratio. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. The use of hhv here is. The power produced by a fuel cell depends. Fuel Cell Hydrogen Consumption Calculation.
From www.linquip.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip Fuel Cell Hydrogen Consumption Calculation The power produced by a fuel cell depends on several factors, including the fuel cell type, size, temperature at which it operates, and pressure. The paper discusses the hydrogen consumption calculation of proton exchange membrane fuel cell for linearly increasing. A compressor and humidifier), the fuel gas supply, thermal. The reaction enthalpy ∆rh is equal to the enthalpy of water. Fuel Cell Hydrogen Consumption Calculation.
From www.marketsandmarkets.com
Hydrogen Future, Trends, Potential and Opportunity in the Hydrogen Fuel Cell Hydrogen Consumption Calculation The use of hhv here is. Typically, the reformate fuel cells operate with hydrogen stoichiometric ratios between 1.1 and 1.2. In the case of the hydrogen fuel cell this value is the gibbs free energy/hhv (237.2 kj/mole ∕ 285.8 kj/mole = 83%). The fuel efficiency, defined as the ratio. A compressor and humidifier), the fuel gas supply, thermal. The paper. Fuel Cell Hydrogen Consumption Calculation.