Heating Curve Of Naphthalene Experiment . The entire experiment could be run in. Once the temperature begins to fall. Naphthalene is in solid state at any temperature below its melting point. When the temperature rises above 90ºc remove the test tube from the water and clamp it. You’ll see the setup for a. To calculate the energy changes that accompany phase changes. The graph above shows the heating curve of naphthalene. Grade 9 advanced science or an introductory chemistry class. Insert a thermometer into the test tube. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). The graph of the heating process. We take advantage of changes between the gas, liquid, and solid states.
from www.numerade.com
The graph above shows the heating curve of naphthalene. You’ll see the setup for a. Insert a thermometer into the test tube. When the temperature rises above 90ºc remove the test tube from the water and clamp it. Naphthalene is in solid state at any temperature below its melting point. The entire experiment could be run in. The graph of the heating process. Once the temperature begins to fall. We take advantage of changes between the gas, liquid, and solid states. To calculate the energy changes that accompany phase changes.
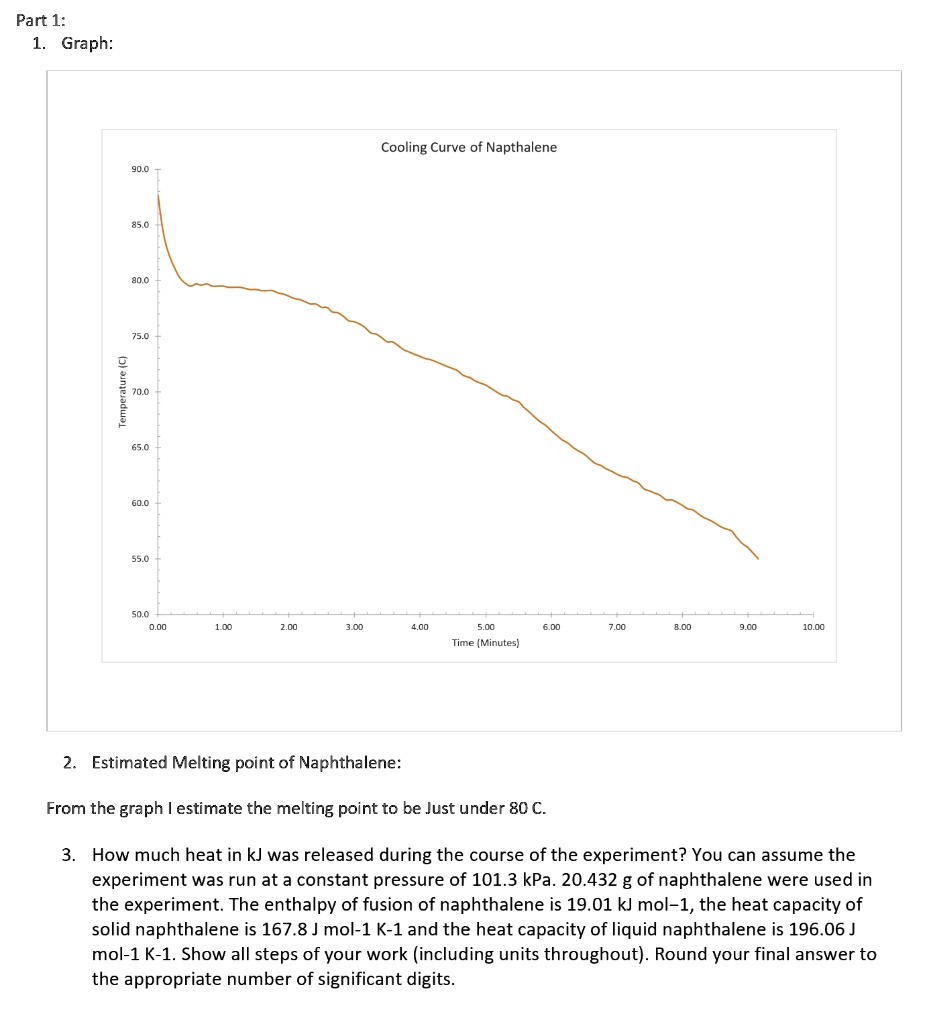
SOLVED Part 1 Graph Cooling Curve of Napthalene 1 1.00 10 Q6 Time
Heating Curve Of Naphthalene Experiment Insert a thermometer into the test tube. To calculate the energy changes that accompany phase changes. Naphthalene is in solid state at any temperature below its melting point. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). Once the temperature begins to fall. You’ll see the setup for a. We take advantage of changes between the gas, liquid, and solid states. Insert a thermometer into the test tube. Grade 9 advanced science or an introductory chemistry class. The graph of the heating process. The graph above shows the heating curve of naphthalene. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). When the temperature rises above 90ºc remove the test tube from the water and clamp it. The entire experiment could be run in.
From ajay-pastel.blogspot.com
Heating Curve / Heating Curve Cie Igcse Chemistry Revision Notes Heating Curve Of Naphthalene Experiment Once the temperature begins to fall. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). The graph above shows the heating curve of naphthalene. When the temperature rises above 90ºc remove the test tube from the water and clamp it. Insert a thermometer into the test tube. We take advantage of changes. Heating Curve Of Naphthalene Experiment.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 1 SPM Chemistry Heating Curve Of Naphthalene Experiment Insert a thermometer into the test tube. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). Naphthalene is in solid state at any temperature below its melting point. To calculate the energy changes that accompany phase changes. The graph of the heating process. Grade 9 advanced science or an introductory chemistry class.. Heating Curve Of Naphthalene Experiment.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curve Of Naphthalene Experiment Once the temperature begins to fall. Insert a thermometer into the test tube. To calculate the energy changes that accompany phase changes. The graph of the heating process. You’ll see the setup for a. We take advantage of changes between the gas, liquid, and solid states. The entire experiment could be run in. The heating curve of naphthalene shows that. Heating Curve Of Naphthalene Experiment.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Of Naphthalene Experiment You’ll see the setup for a. The entire experiment could be run in. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). Naphthalene is in solid state at any temperature below its melting point. To calculate the energy changes that accompany phase changes. Grade 9. Heating Curve Of Naphthalene Experiment.
From ar.inspiredpencil.com
Melting Point Experiment Heating Curve Of Naphthalene Experiment We take advantage of changes between the gas, liquid, and solid states. The graph above shows the heating curve of naphthalene. Naphthalene is in solid state at any temperature below its melting point. Insert a thermometer into the test tube. You’ll see the setup for a. Grade 9 advanced science or an introductory chemistry class. When the temperature rises above. Heating Curve Of Naphthalene Experiment.
From www.youtube.com
Setup for Naphthalene Experiment YouTube Heating Curve Of Naphthalene Experiment Once the temperature begins to fall. We take advantage of changes between the gas, liquid, and solid states. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). Grade 9 advanced science or an introductory chemistry class. The heating curve for carbon dioxide would have only. Heating Curve Of Naphthalene Experiment.
From studylib.net
Heating Curve Lab Heating Curve Of Naphthalene Experiment The entire experiment could be run in. Insert a thermometer into the test tube. When the temperature rises above 90ºc remove the test tube from the water and clamp it. To calculate the energy changes that accompany phase changes. The graph above shows the heating curve of naphthalene. The graph of the heating process. The heating curve for carbon dioxide. Heating Curve Of Naphthalene Experiment.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Heating Curve Of Naphthalene Experiment You’ll see the setup for a. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). Insert a thermometer into the test tube. The graph above shows the heating curve of naphthalene. We take advantage of changes between the gas, liquid, and solid states. To calculate the energy changes that accompany phase changes.. Heating Curve Of Naphthalene Experiment.
From mavink.com
Cooling Curve Of Naphthalene Heating Curve Of Naphthalene Experiment The entire experiment could be run in. The graph of the heating process. The graph above shows the heating curve of naphthalene. We take advantage of changes between the gas, liquid, and solid states. You’ll see the setup for a. Insert a thermometer into the test tube. Once the temperature begins to fall. Naphthalene is in solid state at any. Heating Curve Of Naphthalene Experiment.
From webapi.bu.edu
💋 Freezing point of naphthalene graph. What is the Hypothesis of the Heating Curve Of Naphthalene Experiment Once the temperature begins to fall. When the temperature rises above 90ºc remove the test tube from the water and clamp it. To calculate the energy changes that accompany phase changes. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). Insert a thermometer into the. Heating Curve Of Naphthalene Experiment.
From chemtribe.com
Heating and Cooling Curves ChemTribe Heating Curve Of Naphthalene Experiment Insert a thermometer into the test tube. The graph above shows the heating curve of naphthalene. The graph of the heating process. Naphthalene is in solid state at any temperature below its melting point. You’ll see the setup for a. When the temperature rises above 90ºc remove the test tube from the water and clamp it. The heating curve of. Heating Curve Of Naphthalene Experiment.
From www.studocu.com
Stearic Acid Experiment1 Aim Create a cooling curve for stearic acid Heating Curve Of Naphthalene Experiment You’ll see the setup for a. The graph above shows the heating curve of naphthalene. To calculate the energy changes that accompany phase changes. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). Once the temperature begins to fall. The entire experiment could be run in. The graph of the heating process.. Heating Curve Of Naphthalene Experiment.
From www.slideshare.net
Chapter 2 the structure of the atom Heating Curve Of Naphthalene Experiment The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). You’ll see the setup for a. Insert a thermometer into the test tube. We take advantage of changes between the gas, liquid, and solid states. The graph above shows the heating curve of naphthalene. The entire. Heating Curve Of Naphthalene Experiment.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Of Naphthalene Experiment You’ll see the setup for a. Insert a thermometer into the test tube. Once the temperature begins to fall. We take advantage of changes between the gas, liquid, and solid states. Naphthalene is in solid state at any temperature below its melting point. The graph above shows the heating curve of naphthalene. The heating curve for carbon dioxide would have. Heating Curve Of Naphthalene Experiment.
From www.thinkswap.com
SPM Physics Experiment Heating and cooling of naphthalene Physics Heating Curve Of Naphthalene Experiment The graph of the heating process. We take advantage of changes between the gas, liquid, and solid states. The entire experiment could be run in. To calculate the energy changes that accompany phase changes. The graph above shows the heating curve of naphthalene. When the temperature rises above 90ºc remove the test tube from the water and clamp it. Grade. Heating Curve Of Naphthalene Experiment.
From www.toppr.com
The melting point of naphthalene is 80°C and the room temperature is 30 Heating Curve Of Naphthalene Experiment The entire experiment could be run in. When the temperature rises above 90ºc remove the test tube from the water and clamp it. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). To calculate the energy changes that accompany phase changes. The graph above shows. Heating Curve Of Naphthalene Experiment.
From www.slideshare.net
Chapter 2 heating and cooling of naphthalene Heating Curve Of Naphthalene Experiment Insert a thermometer into the test tube. When the temperature rises above 90ºc remove the test tube from the water and clamp it. The graph above shows the heating curve of naphthalene. The graph of the heating process. We take advantage of changes between the gas, liquid, and solid states. You’ll see the setup for a. Grade 9 advanced science. Heating Curve Of Naphthalene Experiment.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve Of Naphthalene Experiment Grade 9 advanced science or an introductory chemistry class. We take advantage of changes between the gas, liquid, and solid states. Once the temperature begins to fall. The graph above shows the heating curve of naphthalene. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a).. Heating Curve Of Naphthalene Experiment.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curve Of Naphthalene Experiment We take advantage of changes between the gas, liquid, and solid states. Once the temperature begins to fall. Insert a thermometer into the test tube. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). The graph of the heating process. Naphthalene is in solid state at any temperature below its melting point.. Heating Curve Of Naphthalene Experiment.
From www.researchgate.net
TGA curves of naphthalene and mixture of HA/PMMA/naphthalene. Heating Heating Curve Of Naphthalene Experiment Insert a thermometer into the test tube. Naphthalene is in solid state at any temperature below its melting point. Grade 9 advanced science or an introductory chemistry class. When the temperature rises above 90ºc remove the test tube from the water and clamp it. We take advantage of changes between the gas, liquid, and solid states. Once the temperature begins. Heating Curve Of Naphthalene Experiment.
From www.knowledgeboat.com
The melting point of naphthalene is 80°C and the room KnowledgeBoat Heating Curve Of Naphthalene Experiment The graph above shows the heating curve of naphthalene. To calculate the energy changes that accompany phase changes. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). You’ll see the setup for a. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as. Heating Curve Of Naphthalene Experiment.
From heatingwaterdzumekuchi.blogspot.com
Heating Water Heating Water Experiment Heating Curve Of Naphthalene Experiment Insert a thermometer into the test tube. When the temperature rises above 90ºc remove the test tube from the water and clamp it. To calculate the energy changes that accompany phase changes. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). The heating curve for. Heating Curve Of Naphthalene Experiment.
From ethiopialearning.com
Ethiopia Learning Chemistry grade 11 page 146 in English Heating Curve Of Naphthalene Experiment Grade 9 advanced science or an introductory chemistry class. The graph above shows the heating curve of naphthalene. Naphthalene is in solid state at any temperature below its melting point. To calculate the energy changes that accompany phase changes. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). You’ll see the setup. Heating Curve Of Naphthalene Experiment.
From www.slideshare.net
Notechapter2 Heating Curve Of Naphthalene Experiment Grade 9 advanced science or an introductory chemistry class. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). Once the temperature begins to fall. You’ll see the setup for a. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature. Heating Curve Of Naphthalene Experiment.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curve Of Naphthalene Experiment Insert a thermometer into the test tube. When the temperature rises above 90ºc remove the test tube from the water and clamp it. You’ll see the setup for a. To calculate the energy changes that accompany phase changes. The graph above shows the heating curve of naphthalene. Once the temperature begins to fall. The graph of the heating process. Naphthalene. Heating Curve Of Naphthalene Experiment.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 4 SPM Chemistry Heating Curve Of Naphthalene Experiment Grade 9 advanced science or an introductory chemistry class. Once the temperature begins to fall. Insert a thermometer into the test tube. You’ll see the setup for a. The graph above shows the heating curve of naphthalene. The graph of the heating process. Naphthalene is in solid state at any temperature below its melting point. To calculate the energy changes. Heating Curve Of Naphthalene Experiment.
From www.numerade.com
SOLVED Part 1 Graph Cooling Curve of Napthalene 1 1.00 10 Q6 Time Heating Curve Of Naphthalene Experiment When the temperature rises above 90ºc remove the test tube from the water and clamp it. The entire experiment could be run in. To calculate the energy changes that accompany phase changes. We take advantage of changes between the gas, liquid, and solid states. Insert a thermometer into the test tube. The heating curve of naphthalene shows that as heat. Heating Curve Of Naphthalene Experiment.
From brainly.in
The heating curve of a pure substance at one atmosphere pressure is Heating Curve Of Naphthalene Experiment When the temperature rises above 90ºc remove the test tube from the water and clamp it. You’ll see the setup for a. To calculate the energy changes that accompany phase changes. The graph above shows the heating curve of naphthalene. The entire experiment could be run in. The heating curve for carbon dioxide would have only one plateau, at the. Heating Curve Of Naphthalene Experiment.
From www.researchgate.net
Standard curve of Naphthalene solution. Download Scientific Diagram Heating Curve Of Naphthalene Experiment The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). Grade 9 advanced science or an introductory chemistry class. The graph above shows the heating curve of naphthalene. We take advantage of changes between the gas, liquid, and solid states. The entire experiment could be run. Heating Curve Of Naphthalene Experiment.
From study.com
Heating & Cooling Curves Definition, Phases & Examples Lesson Heating Curve Of Naphthalene Experiment The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). Once the temperature begins to fall. The entire experiment could be run in. We take advantage of changes between. Heating Curve Of Naphthalene Experiment.
From www.researchgate.net
Heating curve of hightemperature experiment Download Scientific Diagram Heating Curve Of Naphthalene Experiment The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially rises as particles vibrate more vigorously (a). The graph of the heating process. Insert a thermometer into the test tube. Grade 9 advanced science or an introductory chemistry class. You’ll see the setup for a. We take advantage of changes between the gas,. Heating Curve Of Naphthalene Experiment.
From studylib.net
The graph to the right shows a cooling curve for stearic acid. Stearic Heating Curve Of Naphthalene Experiment Insert a thermometer into the test tube. To calculate the energy changes that accompany phase changes. The graph above shows the heating curve of naphthalene. We take advantage of changes between the gas, liquid, and solid states. Once the temperature begins to fall. The heating curve of naphthalene shows that as heat is added to solid naphthalene, the temperature initially. Heating Curve Of Naphthalene Experiment.
From www.youtube.com
SPM Chemistry Form 4 Heating and cooling of Naphthalene YouTube Heating Curve Of Naphthalene Experiment Naphthalene is in solid state at any temperature below its melting point. When the temperature rises above 90ºc remove the test tube from the water and clamp it. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). You’ll see the setup for a. The heating curve of naphthalene shows that as heat. Heating Curve Of Naphthalene Experiment.
From icolleychemistry.blogspot.com
Ian Colley's Chemistry Blog Brand New Unit! Heating Curve Of Naphthalene Experiment The entire experiment could be run in. The graph of the heating process. The graph above shows the heating curve of naphthalene. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of \(\ce{co_2}\). Naphthalene is in solid state at any temperature below its melting point. We take advantage of changes between the gas, liquid,. Heating Curve Of Naphthalene Experiment.
From www.chegg.com
Solved Cooling curve of pure naphthalene Cooling graph of Heating Curve Of Naphthalene Experiment Once the temperature begins to fall. The graph of the heating process. We take advantage of changes between the gas, liquid, and solid states. Naphthalene is in solid state at any temperature below its melting point. Insert a thermometer into the test tube. Grade 9 advanced science or an introductory chemistry class. The heating curve of naphthalene shows that as. Heating Curve Of Naphthalene Experiment.