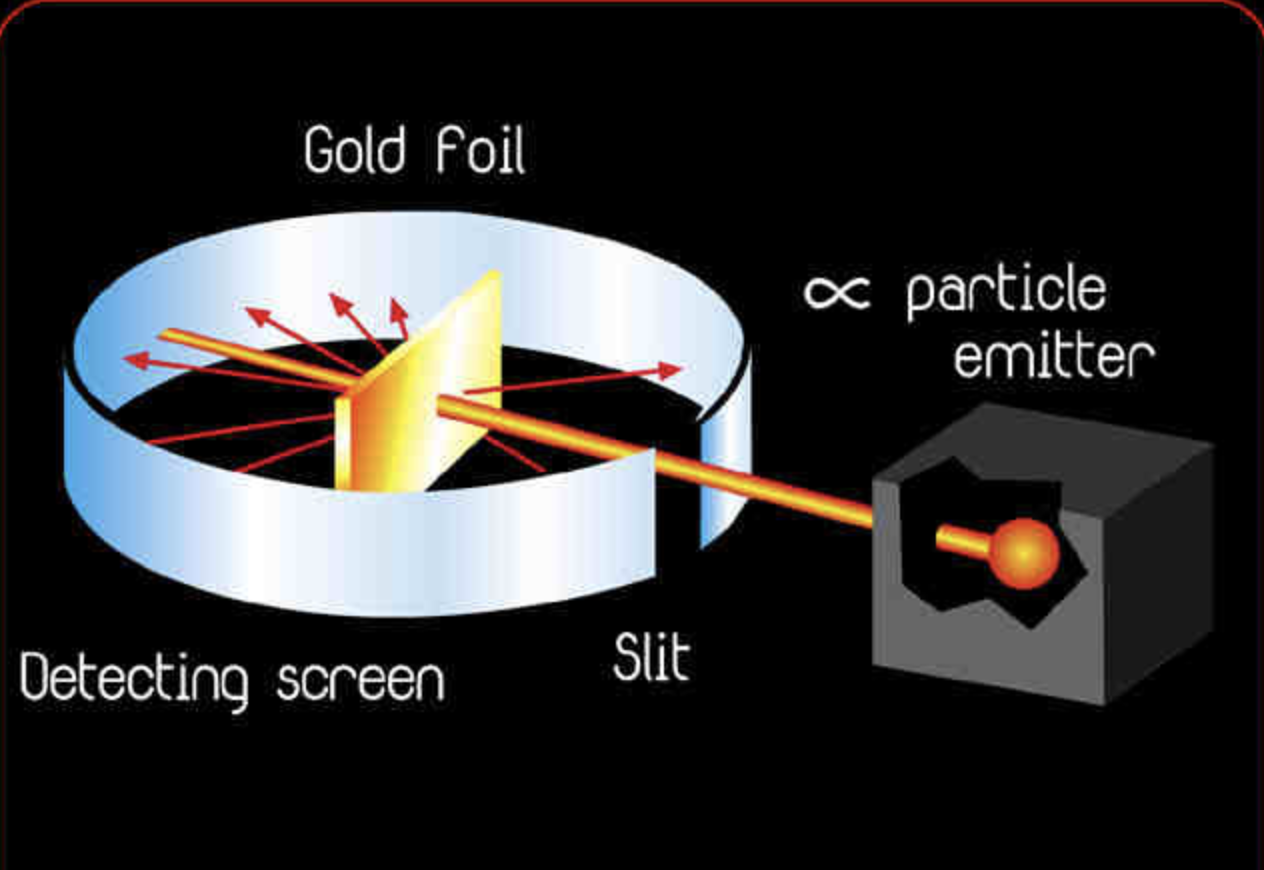
Why Did The Gold Foil Have To Be Thin . a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). They measured each foil's stopping. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? How did rutherford explain the observation. geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum.
from connectstudy.org
in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. How did rutherford explain the observation. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. They measured each foil's stopping.
Ernest Rutherford's Gold Foil Experiment Connect Study
Why Did The Gold Foil Have To Be Thin In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. How did rutherford explain the observation. a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). They measured each foil's stopping.
From www.slideserve.com
PPT Fundamental Atomic Particles Part 1 PowerPoint Presentation, free Why Did The Gold Foil Have To Be Thin They measured each foil's stopping. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. what. Why Did The Gold Foil Have To Be Thin.
From www.slideserve.com
PPT Rutherford’s gold foil experiment PowerPoint Presentation, free Why Did The Gold Foil Have To Be Thin a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? geiger and marsden covered the holes of. Why Did The Gold Foil Have To Be Thin.
From www.slideserve.com
PPT Ernest Rutherford PowerPoint Presentation, free download ID5983179 Why Did The Gold Foil Have To Be Thin geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. In 1911, rutherford and coworkers hans geiger and ernest. Why Did The Gold Foil Have To Be Thin.
From mishiewishieblogg.blogspot.com
Chemistry Gold Foil Experiment Why Did The Gold Foil Have To Be Thin in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served. Why Did The Gold Foil Have To Be Thin.
From www.youtube.com
what did ernest rutherford expect to happen when he aimed a beam of Why Did The Gold Foil Have To Be Thin In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. They measured each foil's stopping. geiger and marsden covered the holes of. Why Did The Gold Foil Have To Be Thin.
From www.slideserve.com
PPT Ernest Rutherford PowerPoint Presentation, free download ID2529914 Why Did The Gold Foil Have To Be Thin in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. what did rutherford observe from shooting. Why Did The Gold Foil Have To Be Thin.
From ernestrutherfordsite.weebly.com
The Gold Foil Experiment Ernest Rutherford Why Did The Gold Foil Have To Be Thin in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). How did rutherford explain the observation. They measured each foil's stopping. geiger and marsden covered the holes of the disc with foils of gold, tin,. Why Did The Gold Foil Have To Be Thin.
From www.expii.com
Gold Foil Experiment — Overview & Importance Expii Why Did The Gold Foil Have To Be Thin a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. in the experiment, rutherford sent a beam of alpha particles (helium nuclei). Why Did The Gold Foil Have To Be Thin.
From www.animalia-life.club
Rutherford Gold Foil Experiment Why Did The Gold Foil Have To Be Thin They measured each foil's stopping. geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? How did rutherford explain the observation. a thin section of gold foil was placed in. Why Did The Gold Foil Have To Be Thin.
From www.youtube.com
Rutherford Gold Foil Experiment My Inter Academy YouTube Why Did The Gold Foil Have To Be Thin They measured each foil's stopping. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. what. Why Did The Gold Foil Have To Be Thin.
From www.expii.com
Gold Foil Experiment — Overview & Importance Expii Why Did The Gold Foil Have To Be Thin geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. They measured each foil's stopping. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? . Why Did The Gold Foil Have To Be Thin.
From www.expii.com
Gold Foil Experiment — Overview & Importance Expii Why Did The Gold Foil Have To Be Thin In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. How did rutherford explain the observation. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum.. Why Did The Gold Foil Have To Be Thin.
From www.youtube.com
Rutherford's Gold Foil Experiment, Explained — Discovering the Nucleus Why Did The Gold Foil Have To Be Thin In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? How did rutherford explain the observation. a thin section of gold foil was placed in front of the slit, and a screen coated with. Why Did The Gold Foil Have To Be Thin.
From www.animalia-life.club
Rutherford Gold Foil Experiment Why Did The Gold Foil Have To Be Thin geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. They measured each foil's stopping. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. . Why Did The Gold Foil Have To Be Thin.
From www.tes.com
Gold foil experiment notes and diagrams Teaching Resources Why Did The Gold Foil Have To Be Thin what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. How did rutherford explain the observation.. Why Did The Gold Foil Have To Be Thin.
From www.animalia-life.club
Rutherford Gold Foil Experiment Why Did The Gold Foil Have To Be Thin How did rutherford explain the observation. a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. what did rutherford observe from shooting. Why Did The Gold Foil Have To Be Thin.
From stock.adobe.com
alpha particles in the rutherford scattering experiment or gold foil Why Did The Gold Foil Have To Be Thin in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. what did rutherford observe from shooting. Why Did The Gold Foil Have To Be Thin.
From study.com
Ernest Rutherford's Gold Foil Experiment Overview & Discovery Why Did The Gold Foil Have To Be Thin In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? They measured each foil's stopping. geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. How. Why Did The Gold Foil Have To Be Thin.
From gcsephysicsninja.com
1. Rutherford's gold foil experiment Why Did The Gold Foil Have To Be Thin They measured each foil's stopping. How did rutherford explain the observation. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). geiger and marsden covered the holes of the disc with foils of gold, tin,. Why Did The Gold Foil Have To Be Thin.
From connectstudy.org
Ernest Rutherford's Gold Foil Experiment Connect Study Why Did The Gold Foil Have To Be Thin in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). How did rutherford explain the observation. a thin section of gold foil was placed in front of the slit, and a screen coated with zinc. Why Did The Gold Foil Have To Be Thin.
From www.animalia-life.club
Rutherford Gold Foil Experiment Why Did The Gold Foil Have To Be Thin In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent. Why Did The Gold Foil Have To Be Thin.
From webapi.bu.edu
Gold foil experiment year. What is the 'Gold Foil Experiment'? The Why Did The Gold Foil Have To Be Thin a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? In 1911, rutherford and coworkers hans geiger and. Why Did The Gold Foil Have To Be Thin.
From www.slideserve.com
PPT Ernest Rutherford & the Gold Foil Experiment PowerPoint Why Did The Gold Foil Have To Be Thin what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. They measured each foil's stopping. How did rutherford. Why Did The Gold Foil Have To Be Thin.
From www.youtube.com
Rutherford's Gold Foil Experiment Or Alpha Rays Scattering Experiment Why Did The Gold Foil Have To Be Thin in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. In 1911, rutherford and coworkers hans geiger. Why Did The Gold Foil Have To Be Thin.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID2260195 Why Did The Gold Foil Have To Be Thin How did rutherford explain the observation. geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). They. Why Did The Gold Foil Have To Be Thin.
From byjus.com
Why did Rutherford select a gold foil in his αray scattering experiment? Why Did The Gold Foil Have To Be Thin what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness. Why Did The Gold Foil Have To Be Thin.
From lzbaehttgs.blogspot.com
Rutherford Gold Foil Experiment, Atomic Structure History Of Atomic Why Did The Gold Foil Have To Be Thin How did rutherford explain the observation. a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold. Why Did The Gold Foil Have To Be Thin.
From askfilo.com
The graph which depicts the results of Rutherford gold foil experiment wi.. Why Did The Gold Foil Have To Be Thin geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. a thin section of gold foil was placed in front of the slit, and a screen coated with zinc sulfide to render it fluorescent served as a counter to detect alpha. In 1911, rutherford and coworkers hans geiger and ernest. Why Did The Gold Foil Have To Be Thin.
From www.youtube.com
Rutherford's Gold Foil Experiment Quick and Simple! YouTube Why Did The Gold Foil Have To Be Thin geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). How did rutherford explain the observation. . Why Did The Gold Foil Have To Be Thin.
From www.bigstockphoto.com
Rutherford Gold Foil Image & Photo (Free Trial) Bigstock Why Did The Gold Foil Have To Be Thin in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? They measured each foil's stopping. In. Why Did The Gold Foil Have To Be Thin.
From readingandwritingprojectcom.web.fc2.com
rutherfords gold foil experiment conclusion Why Did The Gold Foil Have To Be Thin In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. How did rutherford explain the observation. what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? They measured each foil's stopping. a thin section of gold foil was placed in front of the slit,. Why Did The Gold Foil Have To Be Thin.
From www.expii.com
Gold Foil Experiment — Overview & Importance Expii Why Did The Gold Foil Have To Be Thin They measured each foil's stopping. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). How did rutherford explain the observation. a thin section of gold foil was placed in front of the slit, and. Why Did The Gold Foil Have To Be Thin.
From mishiewishieblogg.blogspot.com
Chemistry Gold Foil Experiment Why Did The Gold Foil Have To Be Thin in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms). In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. How did rutherford explain the observation. They measured each foil's stopping.. Why Did The Gold Foil Have To Be Thin.
From wisc.pb.unizin.org
Rutherford’s Experiments (M2Q2) UWMadison Chemistry 103/104 Resource Why Did The Gold Foil Have To Be Thin How did rutherford explain the observation. geiger and marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. They measured each foil's stopping. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking. a thin section of gold foil was placed in front of the slit, and. Why Did The Gold Foil Have To Be Thin.
From www.natureof3laws.co.in
Rutherford Gold Foil Experiment Class 11 Laws Of Nature Why Did The Gold Foil Have To Be Thin what did rutherford observe from shooting thousands and thousands of alpha particles at a thin piece of gold foil? How did rutherford explain the observation. in the experiment, rutherford sent a beam of alpha particles (helium nuclei) emitted from a radioactive source against a thin gold foil (the thickness of about 0.0004 mm, corresponding to about 1000 atoms).. Why Did The Gold Foil Have To Be Thin.