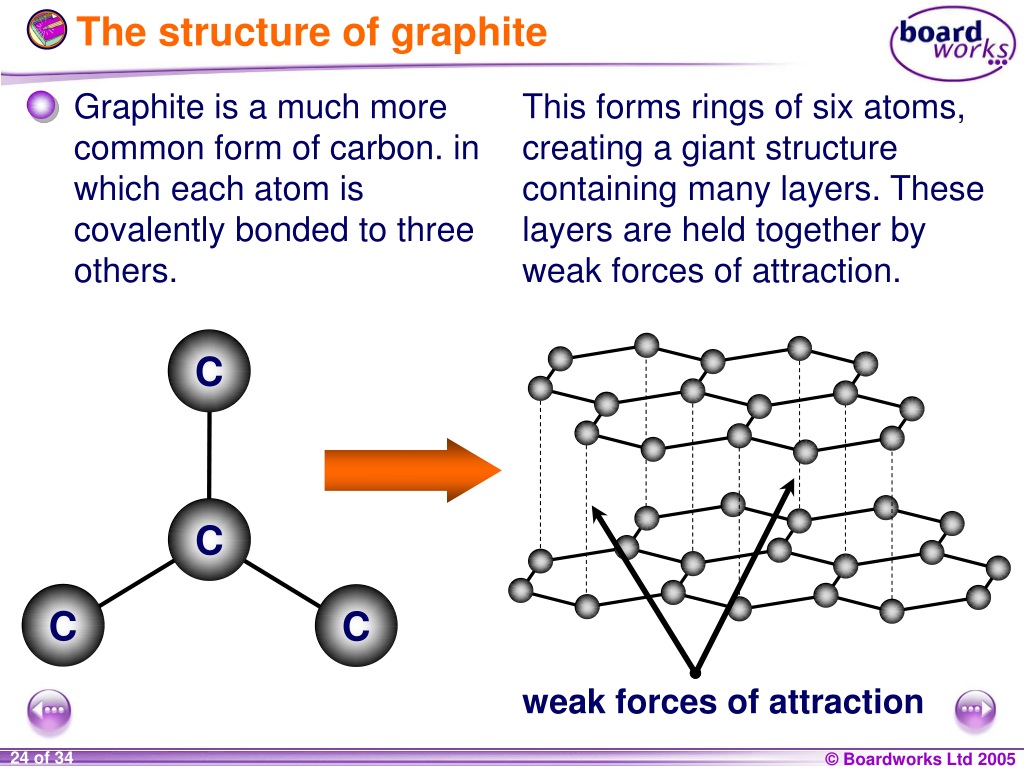
Graphite Is What Type Of Solid . Graphite has a giant covalent structure in which: When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. For example, graphite’s ability to. This page relates the structures of. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Each carbon atom forms three. Covalent bonding a covalent bond is formed by a shared pair of.
from www.slideserve.com
Covalent bonding a covalent bond is formed by a shared pair of. When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Graphite has a giant covalent structure in which: Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Each carbon atom forms three. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. For example, graphite’s ability to. This page relates the structures of.
PPT KS4 Chemistry PowerPoint Presentation, free download ID9350982
Graphite Is What Type Of Solid Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. This page relates the structures of. For example, graphite’s ability to. When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Each carbon atom forms three. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Graphite has a giant covalent structure in which: Covalent bonding a covalent bond is formed by a shared pair of. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions.
From substech.com
Graphite as solid lubricant [SubsTech] Graphite Is What Type Of Solid When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Each carbon atom forms three. Covalent bonding a covalent bond is formed by a shared pair of. Graphite has a giant covalent structure in. Graphite Is What Type Of Solid.
From www.slideshare.net
Graphite Presentation Graphite Is What Type Of Solid For example, graphite’s ability to. When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. Each carbon atom forms three.. Graphite Is What Type Of Solid.
From foundrylexicon.com
Graphite classification chart Graphite Is What Type Of Solid When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Each carbon atom forms three. Graphite has a giant covalent structure in which: For example, graphite’s ability to. Covalent bonding a covalent bond is formed by a shared pair of.. Graphite Is What Type Of Solid.
From www.mdpi.com
Processes Free FullText Graphite Classification Based on Improved Graphite Is What Type Of Solid Covalent bonding a covalent bond is formed by a shared pair of. For example, graphite’s ability to. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together. Graphite Is What Type Of Solid.
From slideplayer.com
Types of Solids SCH 4U1. ppt download Graphite Is What Type Of Solid Covalent bonding a covalent bond is formed by a shared pair of. Each carbon atom forms three. This page relates the structures of. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting. Graphite Is What Type Of Solid.
From www.flickr.com
Graphite (Sri Lanka) 1 Graphite from Ceylon (Sri Lanka). (… Flickr Graphite Is What Type Of Solid Graphite has a giant covalent structure in which: Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Each carbon atom forms three. Covalent bonding a covalent bond is formed by a shared pair of. This page relates the structures of. Each carbon atom in graphite is able to form three covalent bonds to other. Graphite Is What Type Of Solid.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID4500685 Graphite Is What Type Of Solid When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Each carbon atom forms three. Covalent bonding a covalent bond is formed by a shared pair of. For example, graphite’s ability to. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Graphite has a high degree of anisotropy, which. Graphite Is What Type Of Solid.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID4124904 Graphite Is What Type Of Solid Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite has a giant covalent structure in which: Each carbon atom forms three. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Covalent bonding a covalent bond. Graphite Is What Type Of Solid.
From www.askiitians.com
Classification of Crystalline Solids Study Material for IIT JEE Graphite Is What Type Of Solid Each carbon atom forms three. This page relates the structures of. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Covalent bonding a covalent bond is formed by a shared pair of. For example, graphite’s ability to. Covalent network solids are giant covalent substances like diamond, graphite and. Graphite Is What Type Of Solid.
From superiorgraphite.com
About Graphite Graphite Products Graphite Is What Type Of Solid For example, graphite’s ability to. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Graphite has a giant covalent structure in. Graphite Is What Type Of Solid.
From www.sliderbase.com
Properties of Solids Presentation Chemistry Graphite Is What Type Of Solid Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). This page relates the structures of. Each carbon atom forms three. For example, graphite’s ability to. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Covalent bonding a covalent bond is formed by. Graphite Is What Type Of Solid.
From mineralseducationcoalition.org
Graphite Minerals Education Coalition Graphite Is What Type Of Solid Covalent bonding a covalent bond is formed by a shared pair of. Each carbon atom forms three. Graphite has a giant covalent structure in which: When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons,. Graphite Is What Type Of Solid.
From chem.libretexts.org
12.4 The Fundamental Types of Crystalline Solids Chemistry LibreTexts Graphite Is What Type Of Solid When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. Graphite has a giant covalent structure in which: Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming. Graphite Is What Type Of Solid.
From www.slideserve.com
PPT STRUCTURES OF SOLIDS PowerPoint Presentation, free download ID Graphite Is What Type Of Solid When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Covalent bonding a covalent bond is formed by a shared pair of. Each carbon atom forms three. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. For example, graphite’s ability to. Graphite has. Graphite Is What Type Of Solid.
From energyeducation.ca
Graphite Energy Education Graphite Is What Type Of Solid For example, graphite’s ability to. Each carbon atom forms three. Covalent bonding a covalent bond is formed by a shared pair of. Graphite has a giant covalent structure in which: Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Covalent network solids. Graphite Is What Type Of Solid.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Graphite Is What Type Of Solid For example, graphite’s ability to. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. This page relates the structures of. Covalent bonding a covalent bond is formed by a shared pair of. Covalent network solids are giant covalent substances like diamond, graphite. Graphite Is What Type Of Solid.
From grapheneleaderscanada.com
Graphite and Graphene Graphene Leaders Canada Graphite Is What Type Of Solid Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. For example, graphite’s ability to. Covalent bonding a covalent bond is formed by a shared pair of. This page relates the structures of. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting. Graphite Is What Type Of Solid.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Organic Chemistry C6.2i Allotropes of Graphite Is What Type Of Solid Each carbon atom forms three. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Graphite has a giant covalent structure in which: This page relates the structures of. For example, graphite’s ability to. When. Graphite Is What Type Of Solid.
From ar.inspiredpencil.com
Types Of Crystalline Solids Graphite Is What Type Of Solid Covalent bonding a covalent bond is formed by a shared pair of. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. For example, graphite’s ability to. Graphite has. Graphite Is What Type Of Solid.
From studymind.co.uk
Silicon Dioxide, Diamond & Graphite (GCSE Chemistry) Study Mind Graphite Is What Type Of Solid Each carbon atom forms three. Covalent bonding a covalent bond is formed by a shared pair of. For example, graphite’s ability to. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Graphite has. Graphite Is What Type Of Solid.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID6738797 Graphite Is What Type Of Solid Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Covalent network solids are giant covalent substances like diamond, graphite and silicon. Graphite Is What Type Of Solid.
From phys.org
Physicists describe a new type of amorphous solid bodies Graphite Is What Type Of Solid Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. Covalent bonding a covalent bond is formed by a shared pair of. When. Graphite Is What Type Of Solid.
From www.researchgate.net
Structure of graphite. Download Scientific Diagram Graphite Is What Type Of Solid Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. This page relates the structures of. Graphite has a giant covalent structure in which: Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon. Graphite Is What Type Of Solid.
From www.zhycasting.com
Classification of graphite distribution in grey cast iron ZHY Casting Graphite Is What Type Of Solid When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different. Graphite Is What Type Of Solid.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID9350982 Graphite Is What Type Of Solid For example, graphite’s ability to. When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Covalent bonding a covalent bond is formed by a shared pair of. This page relates the structures of. Graphite has a giant covalent structure in. Graphite Is What Type Of Solid.
From www.pinterest.com
PPT Crystalline Solids PowerPoint Presentation, free download ID Graphite Is What Type Of Solid Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Each carbon atom forms three. Graphite has a giant covalent structure in which: Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Covalent bonding a covalent bond is formed by a shared pair. Graphite Is What Type Of Solid.
From www.examplesof.net
Example of Solids Crystalline solids and Amorphous solids Graphite Is What Type Of Solid Graphite has a giant covalent structure in which: This page relates the structures of. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Covalent bonding a covalent bond is formed by a shared pair of. For example, graphite’s ability. Graphite Is What Type Of Solid.
From www.revisechemistry.uk
Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk Graphite Is What Type Of Solid Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Covalent bonding a covalent bond is formed by a shared pair of. Graphite is an exceptional example, composed of planar sheets of covalent crystals that. Graphite Is What Type Of Solid.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID6738797 Graphite Is What Type Of Solid Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Graphite has a giant covalent structure in which: Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Graphite has a high degree of anisotropy, which is caused. Graphite Is What Type Of Solid.
From physicsopenlab.org
Graphite Structure PhysicsOpenLab Graphite Is What Type Of Solid Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. This page relates the structures of. Graphite is an exceptional example, composed of planar sheets of covalent crystals. Graphite Is What Type Of Solid.
From lessonzonevalentina.z13.web.core.windows.net
Types Of Solids In Geometry Graphite Is What Type Of Solid Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per. Graphite Is What Type Of Solid.
From byjus.com
Structure of Graphite with Applications and Crystal Density BYJU'S Graphite Is What Type Of Solid Covalent bonding a covalent bond is formed by a shared pair of. When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. This page relates the structures of. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide (silicon(iv) oxide). Graphite has a high degree of anisotropy, which is caused by two. Graphite Is What Type Of Solid.
From www.slideserve.com
PPT STRUCTURES OF SOLIDS PowerPoint Presentation, free download ID Graphite Is What Type Of Solid For example, graphite’s ability to. Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. Each carbon atom forms three. Graphite has a high degree of anisotropy, which is caused by two types of bonding acting in different crystallographic directions. This page relates. Graphite Is What Type Of Solid.
From commons.wikimedia.org
FileGraphiteanddiamondwithscale.jpg Wikimedia Commons Graphite Is What Type Of Solid When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Graphite is an exceptional example, composed of planar sheets of covalent crystals that are held together in layers by noncovalent forces. Graphite has a giant covalent structure in which: Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms. Graphite Is What Type Of Solid.
From www.slideserve.com
PPT Lecture 2 The Crystal Structure of Solids PowerPoint Presentation Graphite Is What Type Of Solid Each carbon atom in graphite is able to form three covalent bonds to other carbon atoms forming layers of hexagons, leaving one free electron per carbon atom. For example, graphite’s ability to. When carbon material is exposed to high pressure and temperature, natural flake graphite is formed. Covalent network solids are giant covalent substances like diamond, graphite and silicon dioxide. Graphite Is What Type Of Solid.