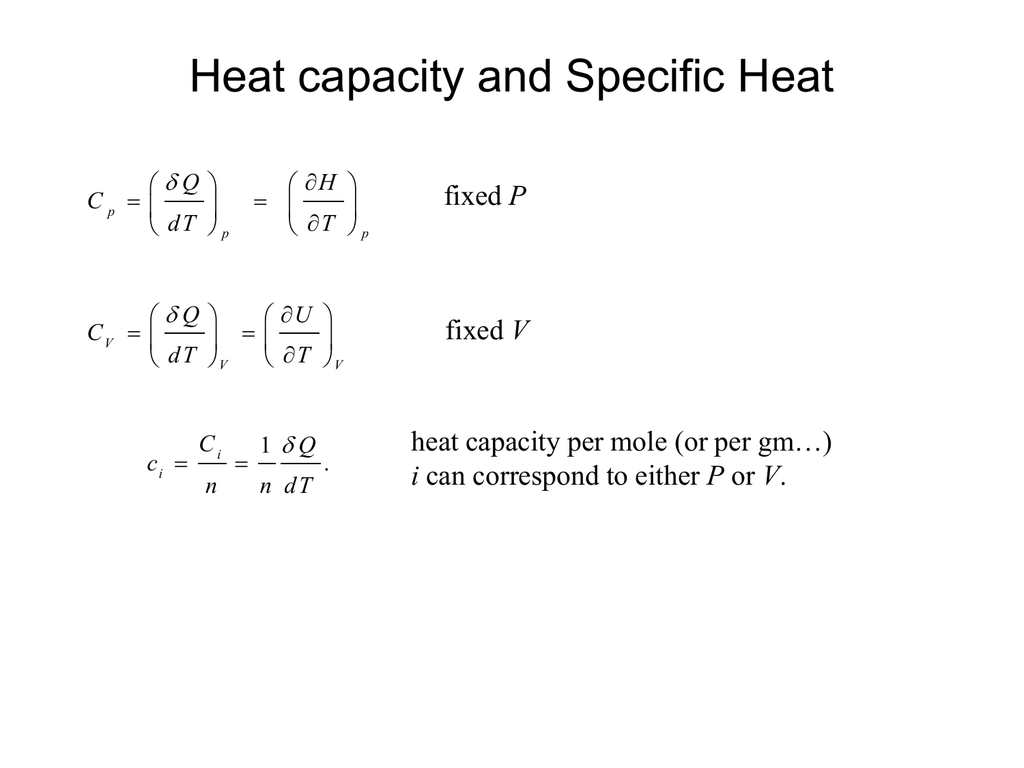What Is Heat Capacity Deduce The Expression Cp-Cv=R . 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at constant volume. Constant volume and constant pressure heat capacities are very important in the calculation of many changes. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. It is useful to have a relationship between these quantities.
from studylib.net
Constant volume and constant pressure heat capacities are very important in the calculation of many changes. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at constant volume. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. It is useful to have a relationship between these quantities. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our.
Lecture_2_specific h..
What Is Heat Capacity Deduce The Expression Cp-Cv=R It is useful to have a relationship between these quantities. Constant volume and constant pressure heat capacities are very important in the calculation of many changes. In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at constant volume. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. It is useful to have a relationship between these quantities.
From www.youtube.com
Specific Heat Capacity Why CpCv=R Molar heat capacity Definition What Is Heat Capacity Deduce The Expression Cp-Cv=R The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Constant volume and constant pressure heat capacities are very important in the calculation of. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.numerade.com
SOLVED Question 3 The specific heat capacities at constant pressure What Is Heat Capacity Deduce The Expression Cp-Cv=R 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. Constant volume and constant pressure heat capacities are very important in the calculation of. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Specific Heat capacities of gas Cp and Cv First law of What Is Heat Capacity Deduce The Expression Cp-Cv=R In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at constant volume. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Relating Heat Capacities Cp and Cv YouTube What Is Heat Capacity Deduce The Expression Cp-Cv=R 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. The heat capacity at constant volume, c v, is the derivative of the internal. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Mayer's formula in Thermodynamics Cp Cv = R proof video in What Is Heat Capacity Deduce The Expression Cp-Cv=R It is useful to have a relationship between these quantities. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Constant volume and. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From studylib.net
Lecture_2_specific h.. What Is Heat Capacity Deduce The Expression Cp-Cv=R Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. Constant volume and constant pressure heat capacities are very important in the calculation of many changes. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature,. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www1.grc.nasa.gov
Specific Heats cp and cv Glenn Research Center NASA What Is Heat Capacity Deduce The Expression Cp-Cv=R Constant volume and constant pressure heat capacities are very important in the calculation of many changes. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From cvcvsc.blogspot.com
Cv And Cp Relationship CVCVSC What Is Heat Capacity Deduce The Expression Cp-Cv=R Constant volume and constant pressure heat capacities are very important in the calculation of many changes. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at constant volume. In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. 47 rows. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From byjus.com
18. Assertion Cp Cv = R Reason R is work done when temperature of What Is Heat Capacity Deduce The Expression Cp-Cv=R 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. It is useful to have a relationship between these quantities. In section 8.1 we. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
3. 11P13.2 CV 2 Specific Heat Capacities of Gases and Mean Free Path What Is Heat Capacity Deduce The Expression Cp-Cv=R The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. It is useful to have a relationship between these quantities. Constant volume and constant. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Class =11 Unit=8 (thermodynamics) The relation between two specific What Is Heat Capacity Deduce The Expression Cp-Cv=R It is useful to have a relationship between these quantities. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. Heat capacity or thermal. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
why Cp is greater than cv Specific Heat Capacity animation hd What Is Heat Capacity Deduce The Expression Cp-Cv=R 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. It is useful to have a relationship between these quantities. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. In section 8.1 we. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From design.udlvirtual.edu.pe
What Is Heat Capacity Prove That Cp Cv R Design Talk What Is Heat Capacity Deduce The Expression Cp-Cv=R In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at constant volume. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.slideserve.com
PPT Thermodynamics I Chapter 2 Properties of Pure Substances What Is Heat Capacity Deduce The Expression Cp-Cv=R Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. In section 8.1 we pointed out that the heat capacity at constant pressure must. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Derive Cp Cv = R Molar heat capacity at constant, pressure volume What Is Heat Capacity Deduce The Expression Cp-Cv=R It is useful to have a relationship between these quantities. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. In section 8.1. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Thermodynamics/ Heat Capacity/ Relationship between Cp and Cv ( Cp Cv What Is Heat Capacity Deduce The Expression Cp-Cv=R 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Constant volume and constant pressure heat capacities are very important in the calculation of many changes. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From quizdialogizes.z4.web.core.windows.net
What Is Cp And Cv In Thermodynamics What Is Heat Capacity Deduce The Expression Cp-Cv=R The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.studypool.com
SOLUTION Heat and First Law of Thermodynamics Prove that Cp Cv = R What Is Heat Capacity Deduce The Expression Cp-Cv=R In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. Constant volume and constant pressure heat capacities are very important in the calculation of many changes. It is useful to have a relationship between these quantities. In section 8.1 we pointed out that the heat capacity at constant pressure must be. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From tipseri.com
What is the relationship between CP CV and R? Tipseri What Is Heat Capacity Deduce The Expression Cp-Cv=R Constant volume and constant pressure heat capacities are very important in the calculation of many changes. It is useful to have a relationship between these quantities. In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. Heat capacity or thermal capacity is described as the quantity of heat essential to alter. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Enthalpy and Specific Heat Derivation Cp = Cv + R YouTube What Is Heat Capacity Deduce The Expression Cp-Cv=R In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at constant volume. In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
RELATION BETWEEN SPECIFIC HEATS Cp and' DERIVE Cp Cv=R YouTube What Is Heat Capacity Deduce The Expression Cp-Cv=R The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. In section 8.1 we pointed out that the heat capacity at constant pressure must. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.numerade.com
SOLVED The heat capacity at constant pressure (Cp) is the partial What Is Heat Capacity Deduce The Expression Cp-Cv=R Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Heat Capacity at Constant Volume (Cv) and at Pressure(Cp) Relation What Is Heat Capacity Deduce The Expression Cp-Cv=R Constant volume and constant pressure heat capacities are very important in the calculation of many changes. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature,. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Why is Cp = Cv + R ?? // Thermodynamics Class 100 YouTube What Is Heat Capacity Deduce The Expression Cp-Cv=R It is useful to have a relationship between these quantities. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. In section 8.1 we. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From askfilo.com
4. Define heat capacity. What are Cp and Cv ? Show that CP −CV =R.A. T.. What Is Heat Capacity Deduce The Expression Cp-Cv=R In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Heat Capacity Relation between Cp and Cv YouTube What Is Heat Capacity Deduce The Expression Cp-Cv=R Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity at constant volume. The heat capacity at constant volume, c v, is the derivative of the. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.slideserve.com
PPT Chapter 17 Thermal Properties PowerPoint Presentation, free What Is Heat Capacity Deduce The Expression Cp-Cv=R In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. Constant volume and constant pressure heat capacities are very important in the calculation of many changes. The heat capacity at constant volume, c v, is the derivative of the internal energy with respect to the temperature, so for our. Heat capacity. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From askfilo.com
452. Define heat capacity. What are Cp and CV ? Show that Cp −Cv =R A) What Is Heat Capacity Deduce The Expression Cp-Cv=R In chapter 7, we derive the relationship between cp c p and cv c v for an ideal gas. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. It is useful to have a relationship between these quantities. 47 rows in thermal physics and thermodynamics,. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Derive Heat Capacity Cp=Cv+R YouTube What Is Heat Capacity Deduce The Expression Cp-Cv=R Constant volume and constant pressure heat capacities are very important in the calculation of many changes. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. In section 8.1 we pointed out that the heat capacity at constant pressure must be greater than the heat capacity. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From design.udlvirtual.edu.pe
What Is Heat Capacity Prove That Cp Cv R Design Talk What Is Heat Capacity Deduce The Expression Cp-Cv=R It is useful to have a relationship between these quantities. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. The heat capacity. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.toppr.com
Cv, respectively, If gamma = CpCv and R is the universal gas constant What Is Heat Capacity Deduce The Expression Cp-Cv=R 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Constant volume and constant pressure heat capacities are very important in the calculation of many changes. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From design.udlvirtual.edu.pe
What Is Heat Capacity Prove That Cp Cv R Design Talk What Is Heat Capacity Deduce The Expression Cp-Cv=R Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. It is useful to have a relationship between these quantities. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. 47 rows in. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From byjus.com
cp/cv=R,how What Is Heat Capacity Deduce The Expression Cp-Cv=R It is useful to have a relationship between these quantities. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. In chapter 7, we. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.youtube.com
Thermodynamics SPECIFIC HEATS cv & cp in 12 Minutes! YouTube What Is Heat Capacity Deduce The Expression Cp-Cv=R 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. In chapter 7, we derive the relationship between cp c p and cv c. What Is Heat Capacity Deduce The Expression Cp-Cv=R.
From www.numerade.com
SOLVED The heat capacity ratio of a gas determines the speed of sound What Is Heat Capacity Deduce The Expression Cp-Cv=R Heat capacity or thermal capacity is described as the quantity of heat essential to alter the temperature of an item by 1 unit. Mayer’s relation (mayer’s law) is the relation between molar heat capacities at constant pressure c p and at constant volume c v for an. It is useful to have a relationship between these quantities. Constant volume and. What Is Heat Capacity Deduce The Expression Cp-Cv=R.