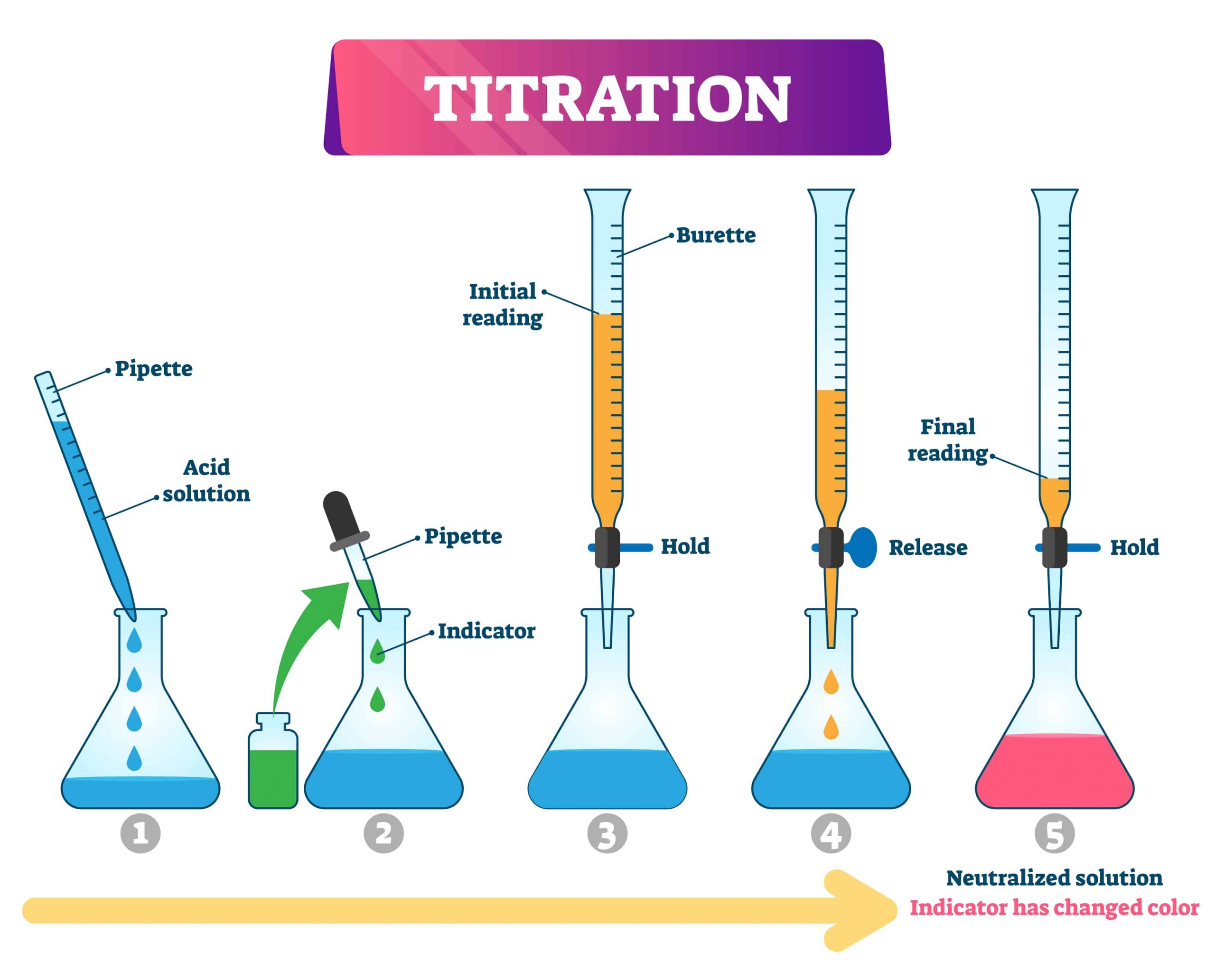
How To Find Indicator For Titration . how titrations work and what you need. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. This page assumes that you know. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Every titration uses the same players: the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint.
from dxoigesvv.blob.core.windows.net
This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. how titrations work and what you need. Every titration uses the same players: The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.
Titration Measuring Device at Amanda Yee blog
How To Find Indicator For Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. how titrations work and what you need. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Every titration uses the same players:
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration How To Find Indicator For Titration Every titration uses the same players: This page assumes that you know. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic. How To Find Indicator For Titration.
From www.youtube.com
Acids and Bases Choosing an Indicator for Titration YouTube How To Find Indicator For Titration Every titration uses the same players: the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. how titrations work and what you need. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The graph shows the results obtained using two indicators (methyl. How To Find Indicator For Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators How To Find Indicator For Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. the titration curves shown in figure. How To Find Indicator For Titration.
From dxozxirwd.blob.core.windows.net
Chemical Indicator In A Titration at Nicholas Terrell blog How To Find Indicator For Titration how titrations work and what you need. This page assumes that you know. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. the titration curves shown in figure 14.20 illustrate the. How To Find Indicator For Titration.
From www.youtube.com
Choosing an INDICATOR for a titration YouTube How To Find Indicator For Titration the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Every titration uses the same players: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base.. How To Find Indicator For Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2976284 How To Find Indicator For Titration the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the indicator changes color at a. How To Find Indicator For Titration.
From mungfali.com
Acid Base Titration Indicator How To Find Indicator For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. how titrations work and what you need. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. How To Find Indicator For Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts How To Find Indicator For Titration This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. Every titration uses the same players: how titrations work and what you need. At the equivalence point. How To Find Indicator For Titration.
From theedge.com.hk
Chemistry How To Titration The Edge How To Find Indicator For Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Every titration uses the same players: how titrations work and what you need. At the equivalence point in a neutralization, the moles of acid. How To Find Indicator For Titration.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog How To Find Indicator For Titration Every titration uses the same players: the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. . How To Find Indicator For Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts How To Find Indicator For Titration how titrations work and what you need. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the indicator changes color at a specific ph, which. How To Find Indicator For Titration.
From www.slideserve.com
PPT Volumetric ANALYSIS/TITRATION PowerPoint Presentation, free How To Find Indicator For Titration the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Every titration uses the same players: . How To Find Indicator For Titration.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration How To Find Indicator For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Every titration uses the same players: This. How To Find Indicator For Titration.
From www.sliderbase.com
Titration How To Find Indicator For Titration the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the titration curves shown in figure. How To Find Indicator For Titration.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog How To Find Indicator For Titration This page assumes that you know. Every titration uses the same players: the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. how titrations work and what you need. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. How To Find Indicator For Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators How To Find Indicator For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained. How To Find Indicator For Titration.
From www.youtube.com
Indicator for titration of strong acid with weak base YouTube How To Find Indicator For Titration This page assumes that you know. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. how titrations work and what you need. Every titration uses the same players: The graph shows the. How To Find Indicator For Titration.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co How To Find Indicator For Titration This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100. How To Find Indicator For Titration.
From saylordotorg.github.io
AcidBase Titrations How To Find Indicator For Titration the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. Every titration uses the same players: This. How To Find Indicator For Titration.
From mungfali.com
Acid Base Titration Indicator How To Find Indicator For Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. how titrations work and what you need. the indicator changes color at a specific ph, which indicates that the reaction has reached its. How To Find Indicator For Titration.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic How To Find Indicator For Titration how titrations work and what you need. Every titration uses the same players: At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak. How To Find Indicator For Titration.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration How To Find Indicator For Titration Every titration uses the same players: the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100. How To Find Indicator For Titration.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog How To Find Indicator For Titration the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. This page assumes that you know. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100. How To Find Indicator For Titration.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog How To Find Indicator For Titration Every titration uses the same players: how titrations work and what you need. This page assumes that you know. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the indicator changes. How To Find Indicator For Titration.
From www.youtube.com
HOW TO DO DOUBLE INDICATOR TITRATION EXPERIMENTS.Chemistry YouTube How To Find Indicator For Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. This page assumes that you know. At. How To Find Indicator For Titration.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE How To Find Indicator For Titration Every titration uses the same players: The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. . How To Find Indicator For Titration.
From www.youtube.com
Strong acidweak base titration indicators YouTube How To Find Indicator For Titration Every titration uses the same players: the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. how titrations work and what you need. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak. How To Find Indicator For Titration.
From mavink.com
Titration Labeled How To Find Indicator For Titration The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. Every titration uses the same players: This. How To Find Indicator For Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent How To Find Indicator For Titration how titrations work and what you need. This page assumes that you know. Every titration uses the same players: the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the. How To Find Indicator For Titration.
From dxoigesvv.blob.core.windows.net
Titration Measuring Device at Amanda Yee blog How To Find Indicator For Titration This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. how titrations work and what you need. Every titration uses the same players: The graph shows the. How To Find Indicator For Titration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 How To Find Indicator For Titration how titrations work and what you need. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m. How To Find Indicator For Titration.
From franco-krussell.blogspot.com
How to Determine Which Indicator to Use for Titration How To Find Indicator For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. how titrations work and what you. How To Find Indicator For Titration.
From exogrvkja.blob.core.windows.net
Indicators For Titrations at Leslie Jackson blog How To Find Indicator For Titration Every titration uses the same players: The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid (acetic acid) with 0.100 m naoh. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. . How To Find Indicator For Titration.
From www.youtube.com
Suitable indicator for titration of strong acid with strong base YouTube How To Find Indicator For Titration At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid. How To Find Indicator For Titration.
From mungfali.com
Endpoint Titration Curve How To Find Indicator For Titration the indicator changes color at a specific ph, which indicates that the reaction has reached its endpoint. how titrations work and what you need. Every titration uses the same players: the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained using two indicators (methyl. How To Find Indicator For Titration.