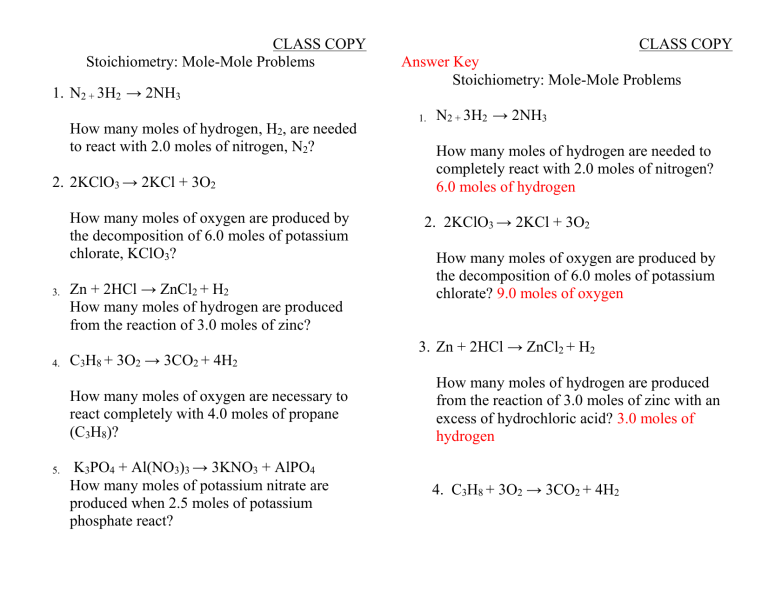
The Mole And Stoichiometry Test Review Answer Key . 1mole = 6.02 × 1023 particles = gram formula mass of. What is the mole ratio of copper to. ♦ avogadro’s number of particles; A) making new bonds between atoms as the old ones are. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. chapter 9 complete stoichiometry review practice problems with answer key.doc. Which one of the following is a definition of a chemical reaction? What is the mole ratio of. Unit 4 motes stoichiometry test review class pd.
from studylib.net
the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. Unit 4 motes stoichiometry test review class pd. chapter 9 complete stoichiometry review practice problems with answer key.doc. A) making new bonds between atoms as the old ones are. Which one of the following is a definition of a chemical reaction? What is the mole ratio of copper to. 1mole = 6.02 × 1023 particles = gram formula mass of. What is the mole ratio of. ♦ avogadro’s number of particles;
Stoichiometry mole to mole answer key
The Mole And Stoichiometry Test Review Answer Key What is the mole ratio of. ♦ avogadro’s number of particles; What is the mole ratio of. chapter 9 complete stoichiometry review practice problems with answer key.doc. Which one of the following is a definition of a chemical reaction? What is the mole ratio of copper to. 1mole = 6.02 × 1023 particles = gram formula mass of. A) making new bonds between atoms as the old ones are. Unit 4 motes stoichiometry test review class pd. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o.
From printablezoneklaudia.z19.web.core.windows.net
Stoichiometry Worksheets Answer Key The Mole And Stoichiometry Test Review Answer Key Which one of the following is a definition of a chemical reaction? What is the mole ratio of. Unit 4 motes stoichiometry test review class pd. ♦ avogadro’s number of particles; A) making new bonds between atoms as the old ones are. the number of moles and mass (in grams) of c 2 h 4 required to react with. The Mole And Stoichiometry Test Review Answer Key.
From omsanomololu.blogspot.com
12+ Chapter 9 Stoichiometry Test Answer Key OmsanOmololu The Mole And Stoichiometry Test Review Answer Key What is the mole ratio of. chapter 9 complete stoichiometry review practice problems with answer key.doc. What is the mole ratio of copper to. Which one of the following is a definition of a chemical reaction? 1mole = 6.02 × 1023 particles = gram formula mass of. A) making new bonds between atoms as the old ones are. Unit. The Mole And Stoichiometry Test Review Answer Key.
From worksheetlist.s3.amazonaws.com
worksheet for basic stoichiometry answers The Mole And Stoichiometry Test Review Answer Key ♦ avogadro’s number of particles; A) making new bonds between atoms as the old ones are. What is the mole ratio of. Unit 4 motes stoichiometry test review class pd. Which one of the following is a definition of a chemical reaction? chapter 9 complete stoichiometry review practice problems with answer key.doc. 1mole = 6.02 × 1023 particles =. The Mole And Stoichiometry Test Review Answer Key.
From studydblang99.z21.web.core.windows.net
Mole To Mole Stoichiometry Worksheet With Answers The Mole And Stoichiometry Test Review Answer Key Unit 4 motes stoichiometry test review class pd. What is the mole ratio of. 1mole = 6.02 × 1023 particles = gram formula mass of. ♦ avogadro’s number of particles; What is the mole ratio of copper to. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55. The Mole And Stoichiometry Test Review Answer Key.
From classdbrose99.z19.web.core.windows.net
Stoichiometry Worksheet Mole To Mole Answers The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. ♦ avogadro’s number of particles; Unit 4 motes stoichiometry test review class pd. chapter 9 complete stoichiometry review practice problems with answer key.doc. What is the mole ratio of. 1mole = 6.02 × 1023 particles = gram formula mass of. Which one of the following is a definition. The Mole And Stoichiometry Test Review Answer Key.
From studylib.net
Stoichiometry Review Answer Key The Mole And Stoichiometry Test Review Answer Key What is the mole ratio of. Which one of the following is a definition of a chemical reaction? A) making new bonds between atoms as the old ones are. ♦ avogadro’s number of particles; chapter 9 complete stoichiometry review practice problems with answer key.doc. the number of moles and mass (in grams) of c 2 h 4 required. The Mole And Stoichiometry Test Review Answer Key.
From studylib.net
Mole Test and stoichiometry version 2 The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. What is the mole ratio of. Unit 4 motes stoichiometry test review class pd. 1mole = 6.02 × 1023 particles = gram formula mass of. What is the mole ratio of copper to. chapter 9 complete stoichiometry review practice problems with answer key.doc. the number of moles. The Mole And Stoichiometry Test Review Answer Key.
From www.englishworksheet.my.id
Stoichiometry Worksheet Answer Key Englishworksheet.my.id The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. ♦ avogadro’s number of particles; Which one of the following is a definition of a chemical reaction? the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. What is the mole. The Mole And Stoichiometry Test Review Answer Key.
From studylib.net
Moles stoichiometry test review The Mole And Stoichiometry Test Review Answer Key chapter 9 complete stoichiometry review practice problems with answer key.doc. 1mole = 6.02 × 1023 particles = gram formula mass of. ♦ avogadro’s number of particles; Unit 4 motes stoichiometry test review class pd. What is the mole ratio of. Which one of the following is a definition of a chemical reaction? the number of moles and mass. The Mole And Stoichiometry Test Review Answer Key.
From mungfali.com
Stoichiometry Practice Worksheet Answer Key The Mole And Stoichiometry Test Review Answer Key Unit 4 motes stoichiometry test review class pd. What is the mole ratio of copper to. chapter 9 complete stoichiometry review practice problems with answer key.doc. What is the mole ratio of. ♦ avogadro’s number of particles; A) making new bonds between atoms as the old ones are. 1mole = 6.02 × 1023 particles = gram formula mass of.. The Mole And Stoichiometry Test Review Answer Key.
From quizzlistroot.z21.web.core.windows.net
Answer Key Stoichiometry Worksheets The Mole And Stoichiometry Test Review Answer Key ♦ avogadro’s number of particles; A) making new bonds between atoms as the old ones are. 1mole = 6.02 × 1023 particles = gram formula mass of. What is the mole ratio of. Unit 4 motes stoichiometry test review class pd. What is the mole ratio of copper to. chapter 9 complete stoichiometry review practice problems with answer key.doc.. The Mole And Stoichiometry Test Review Answer Key.
From www.worksheeto.com
14 Stoichiometry Worksheet 2 Answer Key / The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. Unit 4 motes stoichiometry test review class pd. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. chapter 9 complete stoichiometry review practice problems with answer key.doc. What is. The Mole And Stoichiometry Test Review Answer Key.
From www.worksheeto.com
14 Stoichiometry Worksheet 2 Answer Key / The Mole And Stoichiometry Test Review Answer Key 1mole = 6.02 × 1023 particles = gram formula mass of. A) making new bonds between atoms as the old ones are. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. Unit 4 motes stoichiometry test review class pd. ♦ avogadro’s. The Mole And Stoichiometry Test Review Answer Key.
From danutatilda.blogspot.com
37+ Chapter 11 Stoichiometry Answer Key DanutaTilda The Mole And Stoichiometry Test Review Answer Key What is the mole ratio of copper to. 1mole = 6.02 × 1023 particles = gram formula mass of. A) making new bonds between atoms as the old ones are. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. chapter. The Mole And Stoichiometry Test Review Answer Key.
From learningmagicwirth.z19.web.core.windows.net
Stoichiometry Worksheets With Answer Key The Mole And Stoichiometry Test Review Answer Key ♦ avogadro’s number of particles; 1mole = 6.02 × 1023 particles = gram formula mass of. Which one of the following is a definition of a chemical reaction? the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. chapter 9 complete. The Mole And Stoichiometry Test Review Answer Key.
From answerfullallan101.z19.web.core.windows.net
Mole To Mole Stoichiometry Worksheet Answers The Mole And Stoichiometry Test Review Answer Key What is the mole ratio of. Unit 4 motes stoichiometry test review class pd. 1mole = 6.02 × 1023 particles = gram formula mass of. Which one of the following is a definition of a chemical reaction? ♦ avogadro’s number of particles; What is the mole ratio of copper to. the number of moles and mass (in grams) of. The Mole And Stoichiometry Test Review Answer Key.
From learninglistlang.z19.web.core.windows.net
Chemistry Stoichiometry Worksheet And Answers The Mole And Stoichiometry Test Review Answer Key Which one of the following is a definition of a chemical reaction? A) making new bonds between atoms as the old ones are. What is the mole ratio of copper to. ♦ avogadro’s number of particles; 1mole = 6.02 × 1023 particles = gram formula mass of. What is the mole ratio of. Unit 4 motes stoichiometry test review class. The Mole And Stoichiometry Test Review Answer Key.
From printablezoneklaudia.z19.web.core.windows.net
Stoichiometry Worksheets 1 Mole To Mole Calculations Answer The Mole And Stoichiometry Test Review Answer Key chapter 9 complete stoichiometry review practice problems with answer key.doc. 1mole = 6.02 × 1023 particles = gram formula mass of. A) making new bonds between atoms as the old ones are. What is the mole ratio of. What is the mole ratio of copper to. Which one of the following is a definition of a chemical reaction? ♦. The Mole And Stoichiometry Test Review Answer Key.
From www.pinterest.com
Help your students review Stoichiometry and The Mole using this huge The Mole And Stoichiometry Test Review Answer Key Which one of the following is a definition of a chemical reaction? What is the mole ratio of. chapter 9 complete stoichiometry review practice problems with answer key.doc. What is the mole ratio of copper to. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g. The Mole And Stoichiometry Test Review Answer Key.
From www.worksheeto.com
14 Stoichiometry Worksheet 2 Answer Key / The Mole And Stoichiometry Test Review Answer Key 1mole = 6.02 × 1023 particles = gram formula mass of. A) making new bonds between atoms as the old ones are. What is the mole ratio of. ♦ avogadro’s number of particles; What is the mole ratio of copper to. Which one of the following is a definition of a chemical reaction? Unit 4 motes stoichiometry test review class. The Mole And Stoichiometry Test Review Answer Key.
From printableschooljeff.z21.web.core.windows.net
Molemole Stoichiometry Worksheet With Answers The Mole And Stoichiometry Test Review Answer Key Unit 4 motes stoichiometry test review class pd. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. What is the mole ratio of. 1mole = 6.02 × 1023 particles = gram formula mass of. chapter 9 complete stoichiometry review practice. The Mole And Stoichiometry Test Review Answer Key.
From www.scribd.com
3 Review Stoichiometry Chemistry Practice Quiz and Answers[1] Mole The Mole And Stoichiometry Test Review Answer Key 1mole = 6.02 × 1023 particles = gram formula mass of. A) making new bonds between atoms as the old ones are. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. ♦ avogadro’s number of particles; chapter 9 complete stoichiometry. The Mole And Stoichiometry Test Review Answer Key.
From omsanomololu.blogspot.com
12+ Chapter 9 Stoichiometry Test Answer Key OmsanOmololu The Mole And Stoichiometry Test Review Answer Key Which one of the following is a definition of a chemical reaction? A) making new bonds between atoms as the old ones are. ♦ avogadro’s number of particles; the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. Unit 4 motes stoichiometry. The Mole And Stoichiometry Test Review Answer Key.
From www.scribd.com
key unit 5 stoichiometry test reveiw Mole (Unit) Stoichiometry The Mole And Stoichiometry Test Review Answer Key ♦ avogadro’s number of particles; chapter 9 complete stoichiometry review practice problems with answer key.doc. Unit 4 motes stoichiometry test review class pd. 1mole = 6.02 × 1023 particles = gram formula mass of. What is the mole ratio of. What is the mole ratio of copper to. A) making new bonds between atoms as the old ones are.. The Mole And Stoichiometry Test Review Answer Key.
From learningzonedarlene.z19.web.core.windows.net
Worksheets For Basic Stoichiometry Answer Key The Mole And Stoichiometry Test Review Answer Key the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. chapter 9 complete stoichiometry review practice problems with answer key.doc. What is the mole ratio of copper to. ♦ avogadro’s number of particles; A) making new bonds between atoms as the. The Mole And Stoichiometry Test Review Answer Key.
From studylib.net
Stoichiometry Worksheet with answers The Mole And Stoichiometry Test Review Answer Key chapter 9 complete stoichiometry review practice problems with answer key.doc. 1mole = 6.02 × 1023 particles = gram formula mass of. What is the mole ratio of copper to. ♦ avogadro’s number of particles; Which one of the following is a definition of a chemical reaction? Unit 4 motes stoichiometry test review class pd. the number of moles. The Mole And Stoichiometry Test Review Answer Key.
From studylib.net
Stoichiometry mole to mole answer key The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. ♦ avogadro’s number of particles; chapter 9 complete stoichiometry review practice problems with answer key.doc. Which one of the following. The Mole And Stoichiometry Test Review Answer Key.
From www.onlineworksheet.my.id
Stoichiometry Worksheet Answer Key Onlineworksheet.my.id The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. Which one of the following is a definition of a chemical reaction? Unit 4 motes stoichiometry test review class pd. What is the mole ratio of. chapter 9 complete stoichiometry review practice problems with answer key.doc. 1mole = 6.02 × 1023 particles = gram formula mass of. What. The Mole And Stoichiometry Test Review Answer Key.
From www.scribd.com
Stoichiometry Worksheet+Answers Mole (Unit) Iron The Mole And Stoichiometry Test Review Answer Key What is the mole ratio of. What is the mole ratio of copper to. 1mole = 6.02 × 1023 particles = gram formula mass of. Unit 4 motes stoichiometry test review class pd. ♦ avogadro’s number of particles; the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55. The Mole And Stoichiometry Test Review Answer Key.
From www.templateroller.com
Unit 08 Stoichiometry Worksheet 1 With Answer Key Download The Mole And Stoichiometry Test Review Answer Key the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. What is the mole ratio of. Which one of the following is a definition of a chemical reaction? Unit 4 motes stoichiometry test review class pd. 1mole = 6.02 × 1023 particles. The Mole And Stoichiometry Test Review Answer Key.
From www.pinterest.com
MoleMole Stoichiometry Worksheet Detailed Answer Key Distance The Mole And Stoichiometry Test Review Answer Key chapter 9 complete stoichiometry review practice problems with answer key.doc. Unit 4 motes stoichiometry test review class pd. 1mole = 6.02 × 1023 particles = gram formula mass of. A) making new bonds between atoms as the old ones are. ♦ avogadro’s number of particles; What is the mole ratio of copper to. Which one of the following is. The Mole And Stoichiometry Test Review Answer Key.
From classdbrose99.z19.web.core.windows.net
Stoichiometry Mole To Mole Worksheet Answers The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. Unit 4 motes stoichiometry test review class pd. What is the mole ratio of. ♦ avogadro’s number of particles; What is the mole ratio of copper to. chapter 9 complete stoichiometry review practice problems with answer key.doc. 1mole = 6.02 × 1023 particles = gram formula mass of.. The Mole And Stoichiometry Test Review Answer Key.
From www.scribd.com
Chapter 9 Stoichiometry Test REVIEW SHEET PDF Stoichiometry Mole The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. What is the mole ratio of. Unit 4 motes stoichiometry test review class pd. What is the mole ratio of copper to. chapter 9 complete stoichiometry review practice problems with answer key.doc. Which one of the following is a definition of a chemical reaction? the number of. The Mole And Stoichiometry Test Review Answer Key.
From www.onlineworksheet.my.id
Stoichiometry Worksheet Answer Key Onlineworksheet.my.id The Mole And Stoichiometry Test Review Answer Key 1mole = 6.02 × 1023 particles = gram formula mass of. Unit 4 motes stoichiometry test review class pd. What is the mole ratio of copper to. the number of moles and mass (in grams) of c 2 h 4 required to react with water to produce 9.55 g c 2 h 6 o. ♦ avogadro’s number of particles;. The Mole And Stoichiometry Test Review Answer Key.
From www.pinterest.com
Stoichiometry Mole Mole Problems Worksheet Answers Answers The Mole And Stoichiometry Test Review Answer Key A) making new bonds between atoms as the old ones are. What is the mole ratio of copper to. 1mole = 6.02 × 1023 particles = gram formula mass of. Which one of the following is a definition of a chemical reaction? What is the mole ratio of. Unit 4 motes stoichiometry test review class pd. chapter 9 complete. The Mole And Stoichiometry Test Review Answer Key.