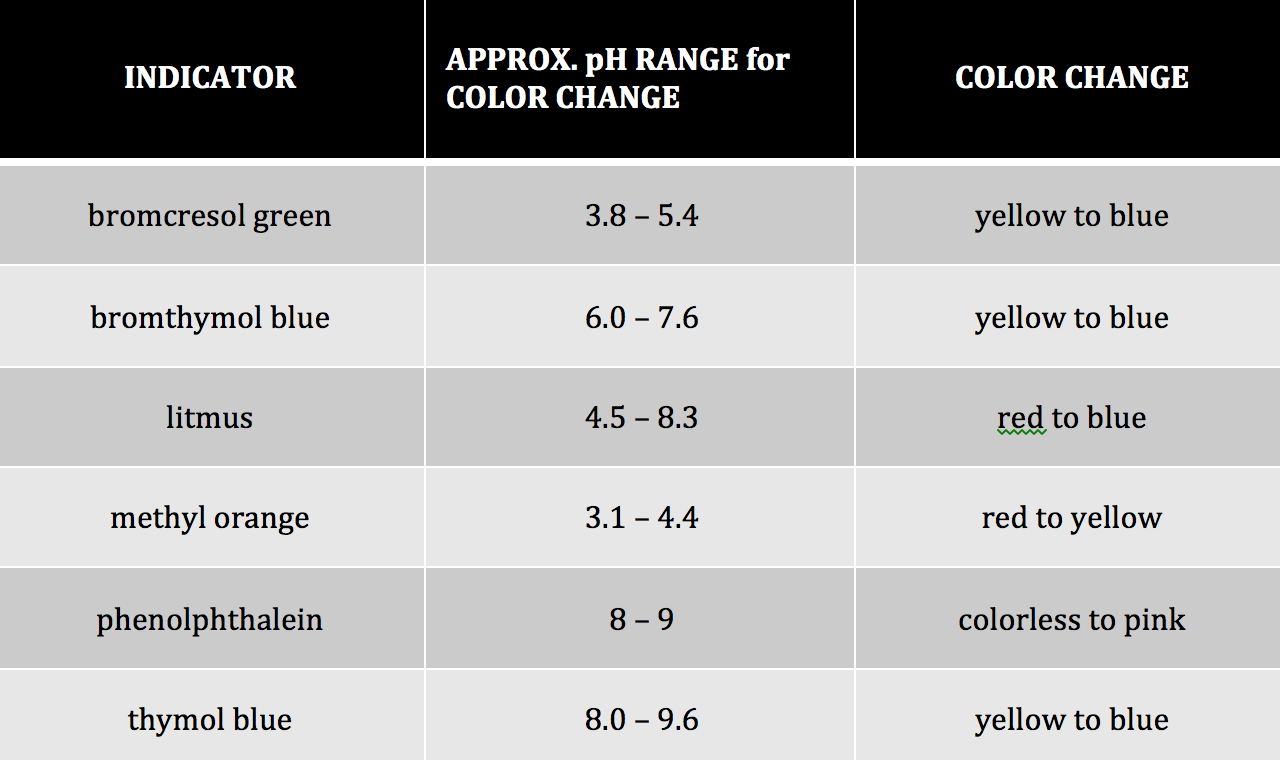
Indicator Science Example . A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. This could be a color change, precipitate. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Here are a few examples of commonly used indicators: These substances are used to determine the acidity or alkalinity of a. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. For precise measurements, a ph meter is used. Ph indicators are used to give a rough value of ph of a chemical solution.
from www.chemedx.org
They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Ph indicators are used to give a rough value of ph of a chemical solution. Here are a few examples of commonly used indicators: For precise measurements, a ph meter is used. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. These substances are used to determine the acidity or alkalinity of a. Indicators are substances whose solutions change color due to changes in ph.
Indicator Activity Assigning Roles for Students in the Laboratory
Indicator Science Example These substances are used to determine the acidity or alkalinity of a. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Ph indicators are used to give a rough value of ph of a chemical solution. This could be a color change, precipitate. Here are a few examples of commonly used indicators: A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. These substances are used to determine the acidity or alkalinity of a. They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. For precise measurements, a ph meter is used.
From www.sciencephoto.com
Universal indicator solution and paper Stock Image C004/7681 Indicator Science Example Ph indicators are used to give a rough value of ph of a chemical solution. These substances are used to determine the acidity or alkalinity of a. For precise measurements, a ph meter is used. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A chemical indicator is a substance that. Indicator Science Example.
From foundoutaboutchemistry.blogspot.com
Found Out About Chemistry Acidbase indicator charts Indicator Science Example A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Ph indicators are used to give a rough value of ph of a chemical solution. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,.. Indicator Science Example.
From www.youtube.com
Essential Science Indicators Introduction YouTube Indicator Science Example An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This could be a color change, precipitate. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Indicators are substances whose solutions change color due to changes in ph. Chemical indicators create a visible sign (usually, a change. Indicator Science Example.
From onstrategyhq.com
27 Examples of Key Performance Indicators OnStrategy Resources Indicator Science Example This could be a color change, precipitate. Indicators are substances whose solutions change color due to changes in ph. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. These substances are used to determine the acidity or alkalinity of a. Chemical indicators create a visible sign (usually, a change in color). Indicator Science Example.
From www.youtube.com
Acids Bases and Salts Class 7 Science Turmeric as a Natural Indicator Indicator Science Example An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. These substances are used to determine the acidity or alkalinity of a.. Indicator Science Example.
From www.youtube.com
Acid Base Indicator Experiment Science Experiment 19 Easy Indicator Science Example These substances are used to determine the acidity or alkalinity of a. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Indicators are substances whose solutions change color due to changes in ph. Here are a few examples of commonly used indicators: Ph indicators are used to give a rough value. Indicator Science Example.
From www.chemedx.org
Indicator Activity Assigning Roles for Students in the Laboratory Indicator Science Example Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Indicators are substances whose solutions change color due to changes in ph. This could be a color change, precipitate. Ph indicators are used to give a rough value of ph of a chemical. Indicator Science Example.
From www.studypool.com
SOLUTION Types of indicator science Studypool Indicator Science Example For precise measurements, a ph meter is used. This could be a color change, precipitate. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Here are a few. Indicator Science Example.
From www.youtube.com
Acids Bases and Indicators in laboratory Science Practical Indicator Science Example We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Here are a few examples of commonly used indicators: For precise measurements, a ph meter is used. They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. This could be a color change, precipitate. Ph. Indicator Science Example.
From www.youtube.com
Natural AcidBase Indicator / School Science Fair Experiment / Basic Indicator Science Example Ph indicators are used to give a rough value of ph of a chemical solution. These substances are used to determine the acidity or alkalinity of a. Here are a few examples of commonly used indicators: We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. An indicator is a weak acid that ionizes within a. Indicator Science Example.
From www.slideserve.com
PPT SECTION I. INDICATORS PowerPoint Presentation, free download ID Indicator Science Example A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Indicators are substances whose solutions change color due to changes in ph. We can represent the protonated form of the indicator molecule as \(\ce{hin}\). Indicator Science Example.
From www.researchgate.net
Important quality indicators in laboratorytheir definition Indicator Science Example These substances are used to determine the acidity or alkalinity of a. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. This could be a color change, precipitate. Here are a few examples of commonly used indicators: An indicator is a weak acid that ionizes within a known ph range, usually. Indicator Science Example.
From www.youtube.com
Indicators Definition, Types, Examples and Facts 10th Class Chemistry Indicator Science Example Indicators are substances whose solutions change color due to changes in ph. For precise measurements, a ph meter is used. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Ph indicators are used to give a rough value of ph of a chemical solution. A chemical indicator is a substance that. Indicator Science Example.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Science Example Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. This could be a color change, precipitate. Here are a few examples of commonly used indicators: They are usually weak acids or bases, but their. For precise measurements, a ph meter is used.. Indicator Science Example.
From study.com
AcidBase Indicators Uses & Examples Lesson Indicator Science Example Ph indicators are used to give a rough value of ph of a chemical solution. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Here are a few examples of commonly used indicators:. Indicator Science Example.
From sciencenotes.org
pH Indicator Chart Colors and Ranges Indicator Science Example These substances are used to determine the acidity or alkalinity of a. Here are a few examples of commonly used indicators: A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. Chemical indicators create a visible sign (usually,. Indicator Science Example.
From ar.inspiredpencil.com
Indicator Science Indicator Science Example Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Here are a few examples of commonly used indicators: These substances are used to determine the acidity or alkalinity of a.. Indicator Science Example.
From www.sciencekiddo.com
7 Red Cabbage Indicator Science Experiments The Science Kiddo Indicator Science Example For precise measurements, a ph meter is used. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. They are usually weak acids or bases, but their. An indicator. Indicator Science Example.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Indicator Science Example For precise measurements, a ph meter is used. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Here are a few examples of commonly used indicators: An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Ph indicators are used to give a. Indicator Science Example.
From fphoto.photoshelter.com
science chemistry acid base universal indicator Fundamental Indicator Science Example Indicators are substances whose solutions change color due to changes in ph. Ph indicators are used to give a rough value of ph of a chemical solution. Here are a few examples of commonly used indicators: An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent the protonated form. Indicator Science Example.
From vhmsscience8.weebly.com
Chemical & Physical Change Indicators 2 VISTA HEIGHTS 8TH GRADE SCIENCE Indicator Science Example Ph indicators are used to give a rough value of ph of a chemical solution. These substances are used to determine the acidity or alkalinity of a. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicators create a visible sign (usually, a change in color) during they detect the. Indicator Science Example.
From www.youtube.com
How To Science Experiment At Home PH Indicator DIY Crafts Tutorial Indicator Science Example Ph indicators are used to give a rough value of ph of a chemical solution. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Here are a few examples of commonly used indicators:. Indicator Science Example.
From www.slideserve.com
PPT Litmus Indicator PowerPoint Presentation, free download ID2790323 Indicator Science Example Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Ph indicators are used to give a rough value of ph of a chemical solution. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units.. Indicator Science Example.
From www.youtube.com
Acids and bases Natural indicators Science Excel YouTube Indicator Science Example We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. This could be a color change, precipitate. They are usually weak acids or bases, but their. For precise measurements, a ph meter is used. These substances are used to determine the acidity or alkalinity of a. Ph indicators are used to give a rough value of. Indicator Science Example.
From www.youtube.com
INDICATORS TURMERIC AS AN INDICATOR SCIENCE CLASS 7 Indicator Science Example Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. These substances are used to determine the acidity or alkalinity of a. An indicator is a weak acid that. Indicator Science Example.
From www.youtube.com
What are Indicators and how do we use them? The Chemistry Journey Indicator Science Example They are usually weak acids or bases, but their. These substances are used to determine the acidity or alkalinity of a. This could be a color change, precipitate. Ph indicators are used to give a rough value of ph of a chemical solution. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. An indicator is. Indicator Science Example.
From mammothmemory.net
There are 2 indicators litmus paper or universal indicator Indicator Science Example A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. An indicator is a weak acid that ionizes within a known ph range, usually about. Indicator Science Example.
From studdy.in
Activity 4.7 NCERT Class 10 Science, Carbon &its Compounds Studdy Indicator Science Example A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This could be a color change, precipitate. For precise measurements, a ph meter is used. Ph indicators are used to give a rough value. Indicator Science Example.
From study.com
AcidBase Indicator Definition, Concept & Examples Lesson Indicator Science Example Here are a few examples of commonly used indicators: We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. These substances are used to determine the acidity or alkalinity of a. For precise measurements, a ph meter is used. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution. Indicator Science Example.
From www.pinterest.com
Colourful Chemistry Chemistry of UNIVERSAL INDICATOR Chemistry Indicator Science Example This could be a color change, precipitate. Ph indicators are used to give a rough value of ph of a chemical solution. They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution. Indicator Science Example.
From www.youtube.com
Science What Are AcidBase Indicators English YouTube Indicator Science Example A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. They are usually weak acids or bases, but their. For precise measurements, a ph meter is used. Indicators are substances whose solutions change color due to changes in ph. Ph indicators are used to give a rough value of ph of a. Indicator Science Example.
From hubpages.com
What is Universal Indicator and How To Use it Indicator Science Example Indicators are substances whose solutions change color due to changes in ph. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. We can represent. Indicator Science Example.
From www.youtube.com
What are Natural Indicators? Class7 Science YouTube Indicator Science Example Indicators are substances whose solutions change color due to changes in ph. For precise measurements, a ph meter is used. Ph indicators are used to give a rough value of ph of a chemical solution. Here are a few examples of commonly used indicators: They are usually weak acids or bases, but their. These substances are used to determine the. Indicator Science Example.
From www.onlinemathlearning.com
Acids and Alkalis (examples, answers, activities, experiment, videos) Indicator Science Example Ph indicators are used to give a rough value of ph of a chemical solution. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. This could be a color change, precipitate. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution change. These substances. Indicator Science Example.
From www.compoundchem.com
The Colours & Chemistry of pH Indicators Compound Interest Indicator Science Example Indicators are substances whose solutions change color due to changes in ph. For precise measurements, a ph meter is used. We can represent the protonated form of the indicator molecule as \(\ce{hin}\) and the. They are usually weak acids or bases, but their. A chemical indicator is a substance that undergoes a distinct observable change when conditions in its solution. Indicator Science Example.