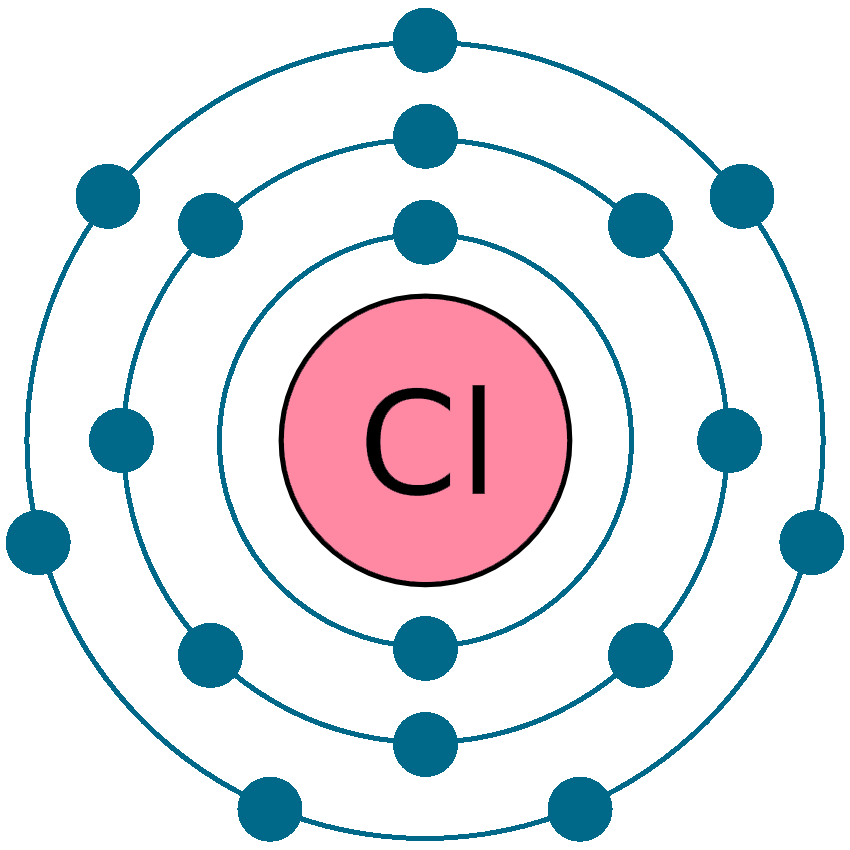
Chlorine Electrical Charge Ion . A neutral chlorine atom has seven electrons in its outermost shell. This atom needs one more electron. 93 rows this is a table of the most common charges for atoms of the chemical elements. 93 rows ionic charge: Anion names work slightly differently than cation names: Charges predict whether an atom bonds with another atom. Chlorine is in group 7 of the periodic table. Most nonmetals become anions when they make ionic compounds. Therefore a chlorine atom has 7 electrons in its outer shell. As a result chlorine is a negative charged ion. The ion formed from a chlorine atom is called a chloride ion. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Here is a chart of element. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6. The neutral atom chlorine (z=17), for instance has 17 electrons.
from www.newtondesk.com
The ion formed from a chlorine atom is called a chloride ion. Here is a chart of element. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. The neutral atom chlorine (z=17), for instance has 17 electrons. This atom needs one more electron. As a result chlorine is a negative charged ion. Therefore a chlorine atom has 7 electrons in its outer shell. Most nonmetals become anions when they make ionic compounds. 93 rows this is a table of the most common charges for atoms of the chemical elements.
Chlorine Cl (Element 17) of Periodic Table Newton Desk
Chlorine Electrical Charge Ion Charges predict whether an atom bonds with another atom. The neutral atom chlorine (z=17), for instance has 17 electrons. Anion names work slightly differently than cation names: 93 rows ionic charge: This electric charge generated on the ion is. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6. Therefore a chlorine atom has 7 electrons in its outer shell. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Chlorine is in group 7 of the periodic table. The ion formed from a chlorine atom is called a chloride ion. This atom needs one more electron. 93 rows this is a table of the most common charges for atoms of the chemical elements. Charges predict whether an atom bonds with another atom. Most nonmetals become anions when they make ionic compounds. Here is a chart of element.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Electrical Charge Ion This atom needs one more electron. As a result chlorine is a negative charged ion. The neutral atom chlorine (z=17), for instance has 17 electrons. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The ion formed from a chlorine atom is called a chloride ion. Anion names work. Chlorine Electrical Charge Ion.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electrical Charge Ion This atom needs one more electron. 93 rows this is a table of the most common charges for atoms of the chemical elements. A neutral chlorine atom has seven electrons in its outermost shell. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Anion names work slightly differently than. Chlorine Electrical Charge Ion.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Electrical Charge Ion Charges predict whether an atom bonds with another atom. As a result chlorine is a negative charged ion. Therefore a chlorine atom has 7 electrons in its outer shell. 93 rows this is a table of the most common charges for atoms of the chemical elements. This electric charge generated on the ion is. Therefore, its ground state electronic configuration. Chlorine Electrical Charge Ion.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Electrical Charge Ion 93 rows ionic charge: Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6. Charges predict whether an atom bonds with another atom. The neutral atom chlorine (z=17), for instance has 17 electrons. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most. Chlorine Electrical Charge Ion.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Electrical Charge Ion This atom needs one more electron. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Charges predict whether an atom bonds with another atom. 93 rows this is a table of the most common charges for atoms of the chemical elements. As a. Chlorine Electrical Charge Ion.
From socratic.org
Question 587a9 Socratic Chlorine Electrical Charge Ion 93 rows ionic charge: Most nonmetals become anions when they make ionic compounds. A neutral chlorine atom has seven electrons in its outermost shell. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Chlorine is in group 7 of the periodic table. Charges. Chlorine Electrical Charge Ion.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Electrical Charge Ion Here is a chart of element. This electric charge generated on the ion is. 93 rows this is a table of the most common charges for atoms of the chemical elements. A neutral chlorine atom has seven electrons in its outermost shell. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion. Chlorine Electrical Charge Ion.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Electrical Charge Ion As a result chlorine is a negative charged ion. Anion names work slightly differently than cation names: Most nonmetals become anions when they make ionic compounds. The neutral atom chlorine (z=17), for instance has 17 electrons. 93 rows this is a table of the most common charges for atoms of the chemical elements. Here is a chart of element. 93. Chlorine Electrical Charge Ion.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce Chlorine Electrical Charge Ion Here is a chart of element. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this is a table of the most common charges for atoms of the chemical elements. Therefore a chlorine atom has 7 electrons in its outer shell. Therefore, its ground state electronic configuration. Chlorine Electrical Charge Ion.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Electrical Charge Ion A neutral chlorine atom has seven electrons in its outermost shell. Anion names work slightly differently than cation names: Chlorine is in group 7 of the periodic table. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. Charges predict whether an atom bonds. Chlorine Electrical Charge Ion.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Electrical Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Charges predict whether an atom bonds with another atom. This atom needs one more electron. The neutral atom chlorine (z=17), for instance has 17 electrons. 93 rows this is a table of the most common charges for atoms of the. Chlorine Electrical Charge Ion.
From mammothmemory.net
Chemical bonding is about atoms achieving full outer shells Chlorine Electrical Charge Ion 93 rows ionic charge: Here is a chart of element. This electric charge generated on the ion is. The ion formed from a chlorine atom is called a chloride ion. This atom needs one more electron. 93 rows this is a table of the most common charges for atoms of the chemical elements. Therefore a chlorine atom has 7 electrons. Chlorine Electrical Charge Ion.
From www.sliderbase.com
Ions Chlorine Electrical Charge Ion This atom needs one more electron. The neutral atom chlorine (z=17), for instance has 17 electrons. A neutral chlorine atom has seven electrons in its outermost shell. As a result chlorine is a negative charged ion. Therefore a chlorine atom has 7 electrons in its outer shell. Chlorine is in group 7 of the periodic table. 93 rows atoms of. Chlorine Electrical Charge Ion.
From delorescgough.blob.core.windows.net
Chlorine Electron Charge at delorescgough blog Chlorine Electrical Charge Ion Here is a chart of element. Anion names work slightly differently than cation names: As a result chlorine is a negative charged ion. Most nonmetals become anions when they make ionic compounds. Therefore a chlorine atom has 7 electrons in its outer shell. This electric charge generated on the ion is. This atom needs one more electron. The neutral atom. Chlorine Electrical Charge Ion.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Electrical Charge Ion Chlorine is in group 7 of the periodic table. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. 93 rows ionic charge: 93 rows this is a table of the most common charges for atoms of the chemical elements. Most nonmetals become anions. Chlorine Electrical Charge Ion.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Chlorine Electrical Charge Ion 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Here is a chart of element. Chlorine is in group 7 of the periodic. Chlorine Electrical Charge Ion.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Electrical Charge Ion Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. This electric charge generated on the ion is. This atom needs one more electron. 93 rows ionic charge:. Chlorine Electrical Charge Ion.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Electrical Charge Ion Therefore a chlorine atom has 7 electrons in its outer shell. This atom needs one more electron. This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Most nonmetals become anions when they make ionic compounds. 93 rows ionic charge: The neutral. Chlorine Electrical Charge Ion.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Electrical Charge Ion 93 rows this is a table of the most common charges for atoms of the chemical elements. Most nonmetals become anions when they make ionic compounds. Anion names work slightly differently than cation names: 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group.. Chlorine Electrical Charge Ion.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chlorine Electrical Charge Ion Charges predict whether an atom bonds with another atom. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6. 93 rows ionic charge: This electric charge generated on the ion is. This atom needs one more electron. 93 rows atoms of the elements display a range of charges, but you can predict the most. Chlorine Electrical Charge Ion.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Chlorine Electrical Charge Ion 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This atom needs one more electron. 93 rows ionic charge: As a result chlorine. Chlorine Electrical Charge Ion.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Electrical Charge Ion Therefore a chlorine atom has 7 electrons in its outer shell. The ion formed from a chlorine atom is called a chloride ion. Here is a chart of element. Most nonmetals become anions when they make ionic compounds. This atom needs one more electron. This electric charge generated on the ion is. Therefore, its ground state electronic configuration can be. Chlorine Electrical Charge Ion.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Electrical Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). The ion formed from a chlorine atom is called a chloride ion. Most nonmetals become anions when they make ionic compounds. This atom needs one more electron. As a result chlorine is a negative charged ion. Chlorine is in group. Chlorine Electrical Charge Ion.
From saylordotorg.github.io
Ions Chlorine Electrical Charge Ion As a result chlorine is a negative charged ion. This electric charge generated on the ion is. Therefore a chlorine atom has 7 electrons in its outer shell. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6. A neutral chlorine atom has seven electrons in its outermost shell. The neutral atom chlorine (z=17),. Chlorine Electrical Charge Ion.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Electrical Charge Ion Chlorine is in group 7 of the periodic table. This electric charge generated on the ion is. 93 rows ionic charge: Most nonmetals become anions when they make ionic compounds. The neutral atom chlorine (z=17), for instance has 17 electrons. As a result chlorine is a negative charged ion. 93 rows atoms of the elements display a range of charges,. Chlorine Electrical Charge Ion.
From slideplayer.com
What are ionic compounds and how do they form? ppt download Chlorine Electrical Charge Ion As a result chlorine is a negative charged ion. Therefore a chlorine atom has 7 electrons in its outer shell. This atom needs one more electron. Charges predict whether an atom bonds with another atom. A neutral chlorine atom has seven electrons in its outermost shell. When the atom loses or gains one or more electrons, the electric charge is. Chlorine Electrical Charge Ion.
From pixels.com
Chlorine Electron Configuration Photograph by Chlorine Electrical Charge Ion 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). As a result chlorine is a negative charged ion. Here is a chart of element. 93 rows this is a table of the most common charges for atoms of the chemical elements. Charges predict whether an. Chlorine Electrical Charge Ion.
From sciencenotes.org
Chlorine Facts Chlorine Electrical Charge Ion As a result chlorine is a negative charged ion. The neutral atom chlorine (z=17), for instance has 17 electrons. This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Anion names work slightly differently than cation names: Charges predict whether an atom. Chlorine Electrical Charge Ion.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chlorine Electrical Charge Ion A neutral chlorine atom has seven electrons in its outermost shell. Therefore a chlorine atom has 7 electrons in its outer shell. This atom needs one more electron. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6. 93 rows ionic charge: Chlorine is in group 7 of the periodic table. 93 rows atoms. Chlorine Electrical Charge Ion.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Chlorine Electrical Charge Ion 93 rows ionic charge: Charges predict whether an atom bonds with another atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. A. Chlorine Electrical Charge Ion.
From slideplayer.com
Ms. Samayoa Birmingham Community Charter High School Chemistry ppt Chlorine Electrical Charge Ion The ion formed from a chlorine atom is called a chloride ion. Most nonmetals become anions when they make ionic compounds. As a result chlorine is a negative charged ion. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element group. A neutral chlorine atom. Chlorine Electrical Charge Ion.
From www.slideserve.com
PPT Ionic Bonds PowerPoint Presentation, free download ID6301347 Chlorine Electrical Charge Ion The ion formed from a chlorine atom is called a chloride ion. The neutral atom chlorine (z=17), for instance has 17 electrons. 93 rows this is a table of the most common charges for atoms of the chemical elements. As a result chlorine is a negative charged ion. 93 rows ionic charge: Most nonmetals become anions when they make ionic. Chlorine Electrical Charge Ion.
From slideplayer.com
Illustrating the bonding of Sodium and Chlorine ppt download Chlorine Electrical Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Charges predict whether an atom bonds with another atom. Therefore a chlorine atom has 7 electrons in its outer shell. 93 rows ionic charge: Anion names work slightly differently than cation names: As a result chlorine is a negative charged. Chlorine Electrical Charge Ion.
From www.youtube.com
How To Determine The Charge of Elements and Ions Chemistry YouTube Chlorine Electrical Charge Ion The neutral atom chlorine (z=17), for instance has 17 electrons. 93 rows this is a table of the most common charges for atoms of the chemical elements. Charges predict whether an atom bonds with another atom. A neutral chlorine atom has seven electrons in its outermost shell. As a result chlorine is a negative charged ion. Anion names work slightly. Chlorine Electrical Charge Ion.
From www.alamy.com
Symbol and electron diagram of chlorine illustration Stock Vector Image Chlorine Electrical Charge Ion Here is a chart of element. 93 rows this is a table of the most common charges for atoms of the chemical elements. The ion formed from a chlorine atom is called a chloride ion. 93 rows atoms of the elements display a range of charges, but you can predict the most common charge of most elements using its element. Chlorine Electrical Charge Ion.