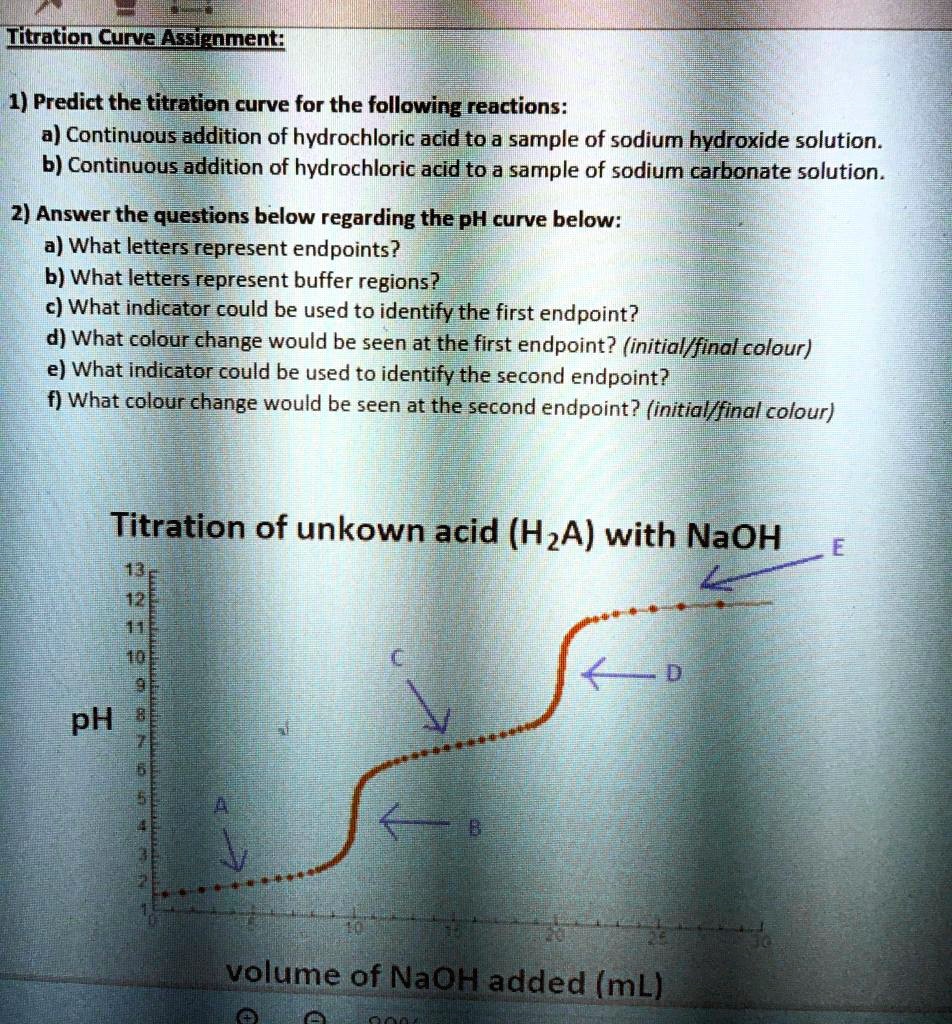
End Point Acid Titration . Reading the volume of titrant added at this point will help us determine the amount. The equivalence point of a. The end point of a titration is the point at which an indicator changes colour at a certain ph. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. Titrations can usually occur in. This should be as close to the equivalence point as possible.
from www.animalia-life.club
Titrations can usually occur in. The equivalence point of a. This should be as close to the equivalence point as possible. Reading the volume of titrant added at this point will help us determine the amount. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. The end point of a titration is the point at which an indicator changes colour at a certain ph. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species.
Endpoint Titration
End Point Acid Titration The end point of a titration is the point at which an indicator changes colour at a certain ph. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. Reading the volume of titrant added at this point will help us determine the amount. Titrations can usually occur in. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. This should be as close to the equivalence point as possible. The end point of a titration is the point at which an indicator changes colour at a certain ph. The equivalence point of a. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below).
From www.animalia-life.club
Endpoint Titration End Point Acid Titration Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. Titrations can usually occur in. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). After the equivalence point, the two curves are identical because the ph is dependent on the excess of. End Point Acid Titration.
From www.sliderbase.com
Titration End Point Acid Titration After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. This should be as close to the equivalence point as possible. The end point of a titration is the point at which an indicator changes colour at a certain ph. Reading the volume of titrant added at. End Point Acid Titration.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID End Point Acid Titration In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. This should be as close to the equivalence point as possible. The equivalence point of a. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. Reading the volume of titrant added at this point will. End Point Acid Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept End Point Acid Titration Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The end point. End Point Acid Titration.
From theedge.com.hk
Chemistry How To Titration The Edge End Point Acid Titration Reading the volume of titrant added at this point will help us determine the amount. Titrations can usually occur in. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. The endpoint for the titration is. End Point Acid Titration.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube End Point Acid Titration After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). Titrations can usually occur in. This should be as close to the equivalence point as. End Point Acid Titration.
From www.youtube.com
AcidBase Titration (Methyl orange end point) YouTube End Point Acid Titration Titrations can usually occur in. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The end point of a titration is the point at which an indicator changes colour at a certain ph. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. The equivalence. End Point Acid Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts End Point Acid Titration Titrations can usually occur in. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. Reading the volume of titrant added at this point will help us determine the amount. After the equivalence point, the two. End Point Acid Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts End Point Acid Titration In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). The end point of a titration is the point at which an indicator changes colour at a certain ph.. End Point Acid Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts End Point Acid Titration In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. This should be as close to the equivalence point as possible. Titrations can usually occur in. The end point of a titration is the point at which an indicator changes colour at a certain ph. After the equivalence point, the two. End Point Acid Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts End Point Acid Titration Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. The equivalence point of a. Titrations can usually occur in. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. The end point of a titration is the point at which an. End Point Acid Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point Acid Titration In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. The equivalence point of a. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in. End Point Acid Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax End Point Acid Titration After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. Titrations can usually occur in. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. The end point of a titration is the point at which an indicator changes colour at a. End Point Acid Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent End Point Acid Titration Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. Titrations can usually occur in. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. This should be as close to the equivalence point as possible. Reading the volume of titrant added. End Point Acid Titration.
From www.animalia-life.club
Endpoint Titration End Point Acid Titration The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. This should be as close to the equivalence point as possible. Titration is used in analytical chemistry to determine. End Point Acid Titration.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration End Point Acid Titration The equivalence point of a. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The end point of a titration is the point at which an indicator. End Point Acid Titration.
From kamora-kjuarez.blogspot.com
What Is an End Point in Titration End Point Acid Titration This should be as close to the equivalence point as possible. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations can usually occur in. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. Reading the volume of titrant added at this point will. End Point Acid Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps End Point Acid Titration The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). The equivalence point of a. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other. End Point Acid Titration.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs End Point Acid Titration The equivalence point of a. The end point of a titration is the point at which an indicator changes colour at a certain ph. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. Titrations can usually occur in. After the equivalence point, the two curves are identical because the ph is dependent on the. End Point Acid Titration.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts End Point Acid Titration Reading the volume of titrant added at this point will help us determine the amount. Titrations can usually occur in. The end point of a titration is the point at which an indicator changes colour at a certain ph. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in. End Point Acid Titration.
From www.slideserve.com
PPT Buffers and Acid/Base Titration PowerPoint Presentation, free End Point Acid Titration In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of a. This should be as close to the equivalence point as possible. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. Titrations can usually occur in. The endpoint for the titration. End Point Acid Titration.
From www.youtube.com
AcidBase Titration (Thymolphthalein end point) YouTube End Point Acid Titration Reading the volume of titrant added at this point will help us determine the amount. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). The end point of a titration is the point at which an indicator changes colour at a certain ph. Titrations can usually occur. End Point Acid Titration.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data End Point Acid Titration Reading the volume of titrant added at this point will help us determine the amount. The equivalence point of a. Titrations can usually occur in. This should be as close to the equivalence point as possible. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. In. End Point Acid Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry End Point Acid Titration Reading the volume of titrant added at this point will help us determine the amount. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). After the equivalence point, the two curves are. End Point Acid Titration.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle End Point Acid Titration After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. This should be as close to the equivalence point as possible. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). Titrations can usually occur. End Point Acid Titration.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point Acid Titration Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below).. End Point Acid Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog End Point Acid Titration After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations can usually occur in. The equivalence point of a. The endpoint for the titration is located right. End Point Acid Titration.
From www.purechemistry.org
ACIDBASE TITRATION Purechemistry End Point Acid Titration The end point of a titration is the point at which an indicator changes colour at a certain ph. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence. End Point Acid Titration.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube End Point Acid Titration In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Titrations can usually occur in. The equivalence point of a. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. This should be as close to the equivalence. End Point Acid Titration.
From schoolbag.info
If the acetic acid being titrated here were replaced by hydrochloric End Point Acid Titration The equivalence point of a. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Reading the volume of titrant added at this point will help us determine the. End Point Acid Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point Acid Titration After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. The end point of a titration is the point at which an indicator changes colour at a certain ph. The equivalence point of a. Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and. End Point Acid Titration.
From ar.inspiredpencil.com
Endpoint Titration End Point Acid Titration Reading the volume of titrant added at this point will help us determine the amount. This should be as close to the equivalence point as possible. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). The end point of a titration is the point at which an. End Point Acid Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators End Point Acid Titration The endpoint for the titration is located right in the middle of this region (marked with a star in the figure below). The equivalence point of a. This should be as close to the equivalence point as possible. Reading the volume of titrant added at this point will help us determine the amount. After the equivalence point, the two curves. End Point Acid Titration.
From mungfali.com
Endpoint Titration Curve End Point Acid Titration Titration is used in analytical chemistry to determine acids, bases, reductants, oxidants and other species. The end point of a titration is the point at which an indicator changes colour at a certain ph. This should be as close to the equivalence point as possible. The equivalence point of a. Reading the volume of titrant added at this point will. End Point Acid Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts End Point Acid Titration This should be as close to the equivalence point as possible. After the equivalence point, the two curves are identical because the ph is dependent on the excess of hydroxide ion in both cases. The equivalence point of a. The endpoint for the titration is located right in the middle of this region (marked with a star in the figure. End Point Acid Titration.