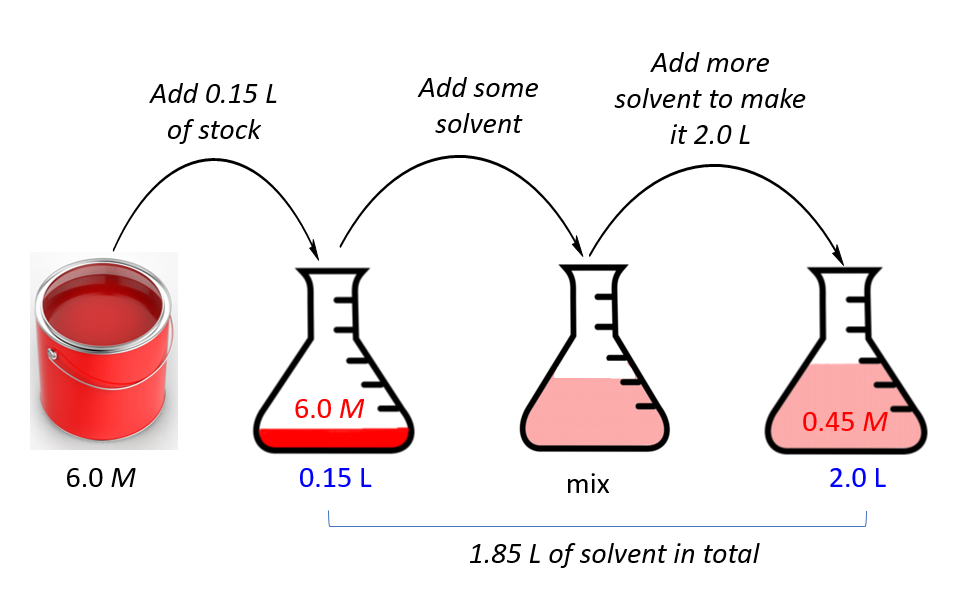
Diluting A Solution Rate Of Reaction . We are often concerned with how much solute is dissolved in a given amount of. Analyse the graph to observe the effect of concentration on the reaction rate. A precipitate will be formed quicker if the. Understand how stock solutions are used in the laboratory. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. The key thing you need to understand here is that. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Place the conical flask on a piece of. Discuss the relationship between the independent variable. The more the solution of a reactant is diluted, the slower the reaction will occur.
from exovgpnzm.blob.core.windows.net
To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. A precipitate will be formed quicker if the. The key thing you need to understand here is that. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Discuss the relationship between the independent variable. The more the solution of a reactant is diluted, the slower the reaction will occur. Analyse the graph to observe the effect of concentration on the reaction rate. Place the conical flask on a piece of. Understand how stock solutions are used in the laboratory.
Dilution And Concentration Of Liquid at Nicole Gardner blog
Diluting A Solution Rate Of Reaction A precipitate will be formed quicker if the. A precipitate will be formed quicker if the. Understand how stock solutions are used in the laboratory. The key thing you need to understand here is that. We are often concerned with how much solute is dissolved in a given amount of. The more the solution of a reactant is diluted, the slower the reaction will occur. To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. Place the conical flask on a piece of. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Analyse the graph to observe the effect of concentration on the reaction rate. Discuss the relationship between the independent variable.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Diluting A Solution Rate Of Reaction We are often concerned with how much solute is dissolved in a given amount of. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. Place the conical flask on a piece of. The key. Diluting A Solution Rate Of Reaction.
From www.youtube.com
Diluting a 12 M stock solution YouTube Diluting A Solution Rate Of Reaction Understand how stock solutions are used in the laboratory. The more the solution of a reactant is diluted, the slower the reaction will occur. We are often concerned with how much solute is dissolved in a given amount of. To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. A precipitate. Diluting A Solution Rate Of Reaction.
From www.numerade.com
Indicate whether each of the following would increase or decrease the Diluting A Solution Rate Of Reaction Place the conical flask on a piece of. Discuss the relationship between the independent variable. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. A precipitate will be formed quicker if the. The key. Diluting A Solution Rate Of Reaction.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Diluting A Solution Rate Of Reaction Analyse the graph to observe the effect of concentration on the reaction rate. A precipitate will be formed quicker if the. Place the conical flask on a piece of. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. To investigate the effect of changing concentration. Diluting A Solution Rate Of Reaction.
From www.slideshare.net
Chemistry Chp 16 Solutions PowerPoint (shortened) Diluting A Solution Rate Of Reaction Place the conical flask on a piece of. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. We are often concerned with how much solute is dissolved in a given amount of. The more the solution of a reactant is diluted, the slower the reaction. Diluting A Solution Rate Of Reaction.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog Diluting A Solution Rate Of Reaction Place the conical flask on a piece of. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Understand how stock solutions are used in the laboratory. To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. Discuss the relationship between the independent. Diluting A Solution Rate Of Reaction.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint Diluting A Solution Rate Of Reaction Understand how stock solutions are used in the laboratory. Discuss the relationship between the independent variable. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. A precipitate will. Diluting A Solution Rate Of Reaction.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Diluting A Solution Rate Of Reaction When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a. Diluting A Solution Rate Of Reaction.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Diluting A Solution Rate Of Reaction This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Analyse the graph to observe the effect of concentration on the reaction rate. We are often concerned with how much solute is dissolved in a given amount of. A precipitate will be formed quicker if the.. Diluting A Solution Rate Of Reaction.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Diluting A Solution Rate Of Reaction This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Analyse the graph to observe the effect of concentration on the reaction rate. The key thing you need to understand here is that. When a dilute acid is added to sodium thiosulphate solution, a pale yellow. Diluting A Solution Rate Of Reaction.
From exovgpnzm.blob.core.windows.net
Dilution And Concentration Of Liquid at Nicole Gardner blog Diluting A Solution Rate Of Reaction The key thing you need to understand here is that. A precipitate will be formed quicker if the. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. We are often concerned with how much. Diluting A Solution Rate Of Reaction.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Diluting A Solution Rate Of Reaction To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. The key thing you need to understand here is that. Discuss the relationship between the independent variable. We are often concerned with how much solute is dissolved in a given amount of. Using a measuring cylinder, add 50 cm 3 of. Diluting A Solution Rate Of Reaction.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Diluting A Solution Rate Of Reaction This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. The more the solution of a reactant is diluted, the slower the reaction will occur. To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. The key. Diluting A Solution Rate Of Reaction.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID Diluting A Solution Rate Of Reaction Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Understand how stock solutions are used in the laboratory. The more the solution of a reactant is diluted, the. Diluting A Solution Rate Of Reaction.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples Diluting A Solution Rate Of Reaction To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. A precipitate will be formed quicker if the. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. Understand how stock solutions are used in the laboratory. We are often concerned with how much. Diluting A Solution Rate Of Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse Diluting A Solution Rate Of Reaction When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Analyse the graph to observe the effect of concentration on the reaction rate. Discuss the relationship between the independent variable. Understand how stock solutions are used in the laboratory. This page explains why changing the concentration changes reaction rates, and illustrates it. Diluting A Solution Rate Of Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse Diluting A Solution Rate Of Reaction We are often concerned with how much solute is dissolved in a given amount of. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. Place the conical flask. Diluting A Solution Rate Of Reaction.
From www.sasadoctor.com
Rate of reaction chemistry igcse Diluting A Solution Rate Of Reaction Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. Analyse the graph to observe the effect of concentration on the reaction rate. The key thing you need to understand here is that. To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. Discuss. Diluting A Solution Rate Of Reaction.
From www.chegg.com
Solved 9 A student uses this apparatus to investigate the Diluting A Solution Rate Of Reaction The more the solution of a reactant is diluted, the slower the reaction will occur. Analyse the graph to observe the effect of concentration on the reaction rate. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. This page explains why changing the concentration changes reaction rates, and illustrates it with. Diluting A Solution Rate Of Reaction.
From www.slideserve.com
PPT DILUTING A SOLUTION PowerPoint Presentation, free download ID Diluting A Solution Rate Of Reaction We are often concerned with how much solute is dissolved in a given amount of. Place the conical flask on a piece of. Understand how stock solutions are used in the laboratory. Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. When a dilute acid is added to sodium thiosulphate solution, a. Diluting A Solution Rate Of Reaction.
From klakbhtvf.blob.core.windows.net
Dilute Solution Has What at Virginia Sebastian blog Diluting A Solution Rate Of Reaction We are often concerned with how much solute is dissolved in a given amount of. Discuss the relationship between the independent variable. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. The more the solution of a reactant is diluted, the slower the reaction will occur. To investigate the effect of. Diluting A Solution Rate Of Reaction.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Diluting A Solution Rate Of Reaction This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Analyse the graph to observe the effect of concentration on the reaction rate. To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. The key thing you. Diluting A Solution Rate Of Reaction.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog Diluting A Solution Rate Of Reaction Understand how stock solutions are used in the laboratory. The more the solution of a reactant is diluted, the slower the reaction will occur. Place the conical flask on a piece of. Discuss the relationship between the independent variable. A precipitate will be formed quicker if the. We are often concerned with how much solute is dissolved in a given. Diluting A Solution Rate Of Reaction.
From www.youtube.com
IGCSE Chemistry Rates of Reaction YouTube Diluting A Solution Rate Of Reaction To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. The more the solution of a reactant is diluted, the slower the reaction will occur. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Understand how. Diluting A Solution Rate Of Reaction.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Diluting A Solution Rate Of Reaction Analyse the graph to observe the effect of concentration on the reaction rate. The more the solution of a reactant is diluted, the slower the reaction will occur. We are often concerned with how much solute is dissolved in a given amount of. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between. Diluting A Solution Rate Of Reaction.
From slideplayer.com
Molecular Theory ppt download Diluting A Solution Rate Of Reaction Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. Understand how stock solutions are used in the laboratory. The key thing you need to understand here is that. We are often concerned with how much solute is dissolved in a given amount of. Place the conical flask on a piece of. This. Diluting A Solution Rate Of Reaction.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Diluting A Solution Rate Of Reaction Place the conical flask on a piece of. Discuss the relationship between the independent variable. The more the solution of a reactant is diluted, the slower the reaction will occur. A precipitate will be formed quicker if the. Analyse the graph to observe the effect of concentration on the reaction rate. When a dilute acid is added to sodium thiosulphate. Diluting A Solution Rate Of Reaction.
From flatworldknowledge.lardbucket.org
Factors That Affect Reaction Rates Diluting A Solution Rate Of Reaction Analyse the graph to observe the effect of concentration on the reaction rate. A precipitate will be formed quicker if the. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of. Diluting A Solution Rate Of Reaction.
From www.slideshare.net
Rate Of Reaction Diluting A Solution Rate Of Reaction Using a measuring cylinder, add 50 cm 3 of dilute sodium thiosulfate solution to a conical flask. Understand how stock solutions are used in the laboratory. We are often concerned with how much solute is dissolved in a given amount of. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. To. Diluting A Solution Rate Of Reaction.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Diluting A Solution Rate Of Reaction When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Discuss the relationship between the independent variable. Understand how stock solutions are used in the laboratory. Place the conical flask on a piece of. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute. Diluting A Solution Rate Of Reaction.
From exylrixiz.blob.core.windows.net
Elution Vs Dilution at Araceli Tann blog Diluting A Solution Rate Of Reaction The key thing you need to understand here is that. The more the solution of a reactant is diluted, the slower the reaction will occur. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. Discuss the relationship between the independent variable. A precipitate will be. Diluting A Solution Rate Of Reaction.
From www.youtube.com
Dilution, solution, ratio, proportion (laboratory. calculations) YouTube Diluting A Solution Rate Of Reaction To investigate the effect of changing concentration on the rate of reaction by measuring the formation of a precipitate. Place the conical flask on a piece of. Analyse the graph to observe the effect of concentration on the reaction rate. Understand how stock solutions are used in the laboratory. A precipitate will be formed quicker if the. This page explains. Diluting A Solution Rate Of Reaction.
From www.youtube.com
CHEMISTRY 101 Calculating Ion Concentration by Molarity and Solution Diluting A Solution Rate Of Reaction Understand how stock solutions are used in the laboratory. Analyse the graph to observe the effect of concentration on the reaction rate. Place the conical flask on a piece of. A precipitate will be formed quicker if the. The key thing you need to understand here is that. When a dilute acid is added to sodium thiosulphate solution, a pale. Diluting A Solution Rate Of Reaction.
From www.youtube.com
Expt 2 reaction rates dilution YouTube Diluting A Solution Rate Of Reaction This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction between dilute hydrochloric acid and sodium thiosulfate solution. We are often concerned with how much solute is dissolved in a given amount of. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Discuss the relationship. Diluting A Solution Rate Of Reaction.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Diluting A Solution Rate Of Reaction Understand how stock solutions are used in the laboratory. The key thing you need to understand here is that. When a dilute acid is added to sodium thiosulphate solution, a pale yellow precipitate of sulphur is formed. Discuss the relationship between the independent variable. This page explains why changing the concentration changes reaction rates, and illustrates it with the reaction. Diluting A Solution Rate Of Reaction.