Consider A Galvanic Cell In Which Al3+ . consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. Describe the basic components of galvanic cells. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Use cell notation to describe galvanic cells.
from www.chegg.com
Describe the basic components of galvanic cells. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Use cell notation to describe galvanic cells. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium.
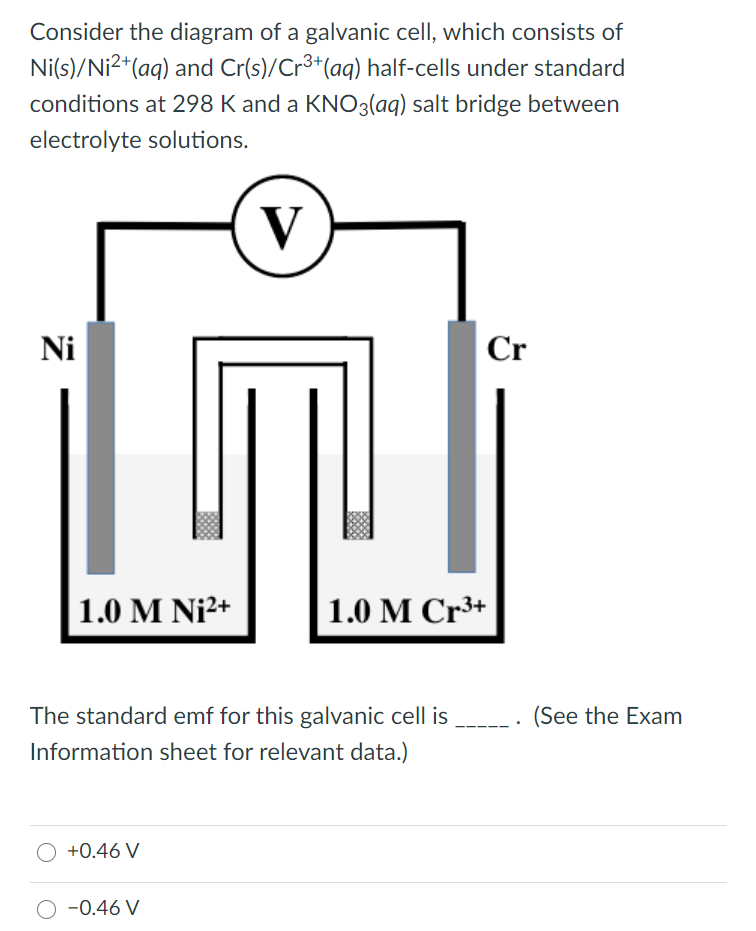
Solved Consider the diagram of a galvanic cell, which
Consider A Galvanic Cell In Which Al3+ In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. Use cell notation to describe galvanic cells. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Describe the basic components of galvanic cells. Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3.
From www.chegg.com
Solved Consider the following galvanic cell that uses the Consider A Galvanic Cell In Which Al3+ a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. Use cell notation to. Consider A Galvanic Cell In Which Al3+.
From www.numerade.com
SOLVED Consider the semireactions and the respective potentials for Consider A Galvanic Cell In Which Al3+ Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. consider a different galvanic cell (see figure \(\pageindex{3}\)). Consider A Galvanic Cell In Which Al3+.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Consider A Galvanic Cell In Which Al3+ Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Use cell notation to describe galvanic cells. Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium.. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved Consider a galvanic cell in which Al3+ is reduced to Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. a galvanic. Consider A Galvanic Cell In Which Al3+.
From exyedkqyr.blob.core.windows.net
A Galvanic Cell Converts at Celia Bosworth blog Consider A Galvanic Cell In Which Al3+ consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. Use cell notation. Consider A Galvanic Cell In Which Al3+.
From www.studypool.com
SOLUTION Galvanic cell their representation Studypool Consider A Galvanic Cell In Which Al3+ Describe the basic components of galvanic cells. Use cell notation to describe galvanic cells. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. In this cell, a solid magnesium anode is immersed in an. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved Consider a galvanic cell in which Al3+ is reduced to Consider A Galvanic Cell In Which Al3+ consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions:. Consider A Galvanic Cell In Which Al3+.
From www.numerade.com
SOLVED 'Consider a galvanic cell consisting of Fe?+ + 2e" 5 Fe E"red Consider A Galvanic Cell In Which Al3+ In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. Use cell notation to describe galvanic cells. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. consider a. Consider A Galvanic Cell In Which Al3+.
From www.numerade.com
SOLVED Consider Galvanic cell that operates spontaneously summarized Consider A Galvanic Cell In Which Al3+ consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. Use cell notation to describe galvanic cells. Describe the basic components of galvanic cells. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Consider a galvanic cell in which al3+al3+ is reduced. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved . C Consider a galvanic cell in which Al3+ is Consider A Galvanic Cell In Which Al3+ Use cell notation to describe galvanic cells. Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved ?∞ Consider a galvanic cell in which Al3+ is Consider A Galvanic Cell In Which Al3+ Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. consider a galvanic cell in which al3+ is. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Consider the galvanic cell shown belowThe atomic Consider A Galvanic Cell In Which Al3+ consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Describe the basic components of galvanic cells. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. Consider. Consider A Galvanic Cell In Which Al3+.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Consider A Galvanic Cell In Which Al3+ In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. consider a different galvanic cell (see figure \(\pageindex{3}\)) based. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved Consider a galvanic cell in which A13+ is reduced to Consider A Galvanic Cell In Which Al3+ In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. Use cell notation to describe galvanic cells. consider. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved Consider the following galvanic cell Al(s) Al^3+ Consider A Galvanic Cell In Which Al3+ In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. Consider a galvanic cell in which al3+ is reduced to elemental. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved Consider a galvanic cell in which Al3+Al3+ is Consider A Galvanic Cell In Which Al3+ a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. consider a galvanic cell in which. Consider A Galvanic Cell In Which Al3+.
From www.coursehero.com
[Solved] 5. A galvanic cell uses Al(s) and Al3+(aq) in one compartment Consider A Galvanic Cell In Which Al3+ Use cell notation to describe galvanic cells. Describe the basic components of galvanic cells. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. consider a galvanic cell in which al3+ is reduced to. Consider A Galvanic Cell In Which Al3+.
From chem.eduinsightful.com
Galvanic Cells Decoded Igniting a Spark of Knowledge Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Use cell notation to describe galvanic cells. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii.. Consider A Galvanic Cell In Which Al3+.
From exywnyysk.blob.core.windows.net
The Galvanic Cell Reaction at Darin Koch blog Consider A Galvanic Cell In Which Al3+ consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. In this cell, a solid magnesium anode is immersed in. Consider A Galvanic Cell In Which Al3+.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. In this cell, a solid magnesium anode. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
2. Consider a galvanic cell in which Al3+ is reduced Consider A Galvanic Cell In Which Al3+ Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. Describe the basic components of galvanic cells.. Consider A Galvanic Cell In Which Al3+.
From www.numerade.com
SOLVED For the galvanic cell represented by the reaction, Al(s) + Cd2 Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. consider a galvanic cell in which. Consider A Galvanic Cell In Which Al3+.
From www.numerade.com
SOLVED Consider a galvanic cell based on the reaction Al3+(aq) + Mg(s Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Use cell notation to describe galvanic cells. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. consider a galvanic cell in which al3+ is reduced to elemental. Consider A Galvanic Cell In Which Al3+.
From solvedlib.com
Consider the diagram of a galvanic cell, which consis… SolvedLib Consider A Galvanic Cell In Which Al3+ Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. Use cell notation to describe galvanic cells. a. Consider A Galvanic Cell In Which Al3+.
From www.geeksforgeeks.org
What is Cell Potential Definition, Formula, and Examples Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Describe the basic components of galvanic cells. In this cell, a solid magnesium anode is immersed in an aqueous. Consider A Galvanic Cell In Which Al3+.
From www.slideserve.com
PPT Chemistry 142 Chapter 18 Electrochemistry PowerPoint Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via. Consider A Galvanic Cell In Which Al3+.
From www.numerade.com
SOLVED Use the image of the galvanic cell at the right and the Consider A Galvanic Cell In Which Al3+ Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. Use cell notation to describe galvanic cells. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Describe the. Consider A Galvanic Cell In Which Al3+.
From brainly.com
Galvanic cell runs on the following reaction Al (s) + Fe3+ (aq) → Al3 Consider A Galvanic Cell In Which Al3+ Consider a galvanic cell in which al3+al3+ is reduced to elemental aluminum and magnesium. Describe the basic components of galvanic cells. consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: Use cell notation to describe galvanic cells. consider a galvanic cell in which al3+ is reduced to. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved Consider the diagram of a galvanic cell, which Consider A Galvanic Cell In Which Al3+ consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. Consider a galvanic cell in which al3+al3+ is reduced to elemental. Consider A Galvanic Cell In Which Al3+.
From courses.lumenlearning.com
Galvanic Cells Chemistry Consider A Galvanic Cell In Which Al3+ Describe the basic components of galvanic cells. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved A voltaic cell is constructed from a standard Al3+Al Consider A Galvanic Cell In Which Al3+ Describe the basic components of galvanic cells. consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is. In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. Consider a galvanic cell. Consider A Galvanic Cell In Which Al3+.
From www.vrogue.co
Electrochemistry Part 2 Galvanic Cell Reduction Poten vrogue.co Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Consider. Consider A Galvanic Cell In Which Al3+.
From www.numerade.com
SOLVED Question 15 of 20 Attempt 1 Consider a galvanic cell in which Consider A Galvanic Cell In Which Al3+ consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg²+. Consider a galvanic cell in which al3+ is reduced to elemental aluminum and magnesium metal is oxidized to mg2+. Describe the basic components of galvanic cells. In this cell, a solid magnesium anode is immersed in an aqueous solution of. Consider A Galvanic Cell In Which Al3+.
From www.numerade.com
SOLVED Consider a voltaic cell based on the following reaction Al3 Consider A Galvanic Cell In Which Al3+ consider a different galvanic cell (see figure \(\pageindex{3}\)) based on the spontaneous reaction between solid magnesium and aqueous iron(iii) ions: In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. a galvanic cell based on the. Consider A Galvanic Cell In Which Al3+.
From www.chegg.com
Solved Consider a galvanic cell consisting of Consider A Galvanic Cell In Which Al3+ In this cell, a solid magnesium anode is immersed in an aqueous solution of magnesium chloride that is connected via a salt bridge to an aqueous solution containing a mixture of iron(iii. a galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. consider a galvanic cell in which al3+ is reduced. Consider A Galvanic Cell In Which Al3+.